2022
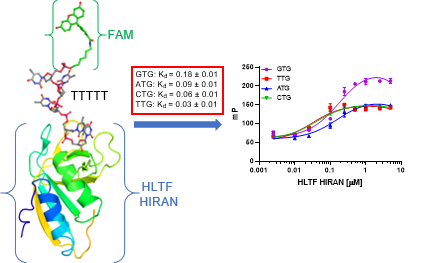
73. Dusek, C. O.; Dash, R. C.; McPherson, K. S.; Calhoun, J. T.; Bezsonova, I.; Korzhnev, D. M.; Hadden, M. K. DNA sequence specificity reveals a role of the HLTF HIRAN domain in the recognition of trinucleotide repeats. Biochemistry 2022, 61, 992-1004.
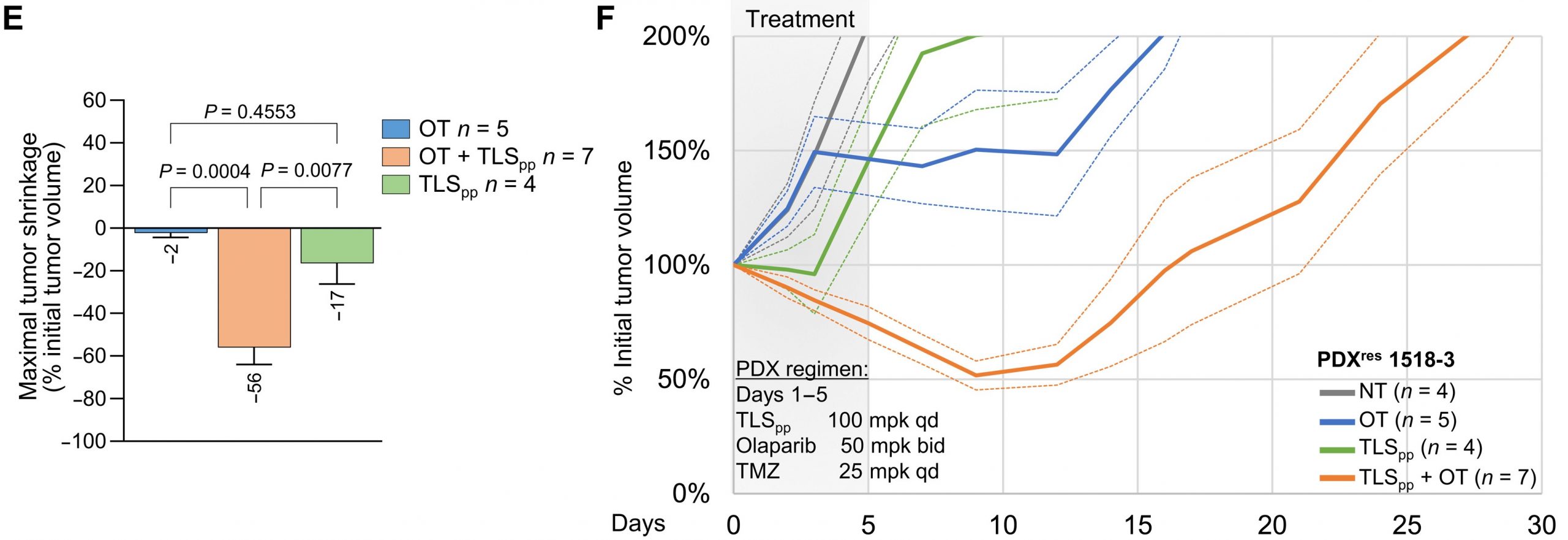
72. Stanzione, M.; Zhong, J.; Wong, E.; Lasalle, T. J.; Wise, J. F.; Simoneau, A.; Myers, D. T.; Phat, S.; Sade-Feldman, M.; Lawrence, M. S.; Hadden, M. K.; Zou, L.; Farago, S.; Dyson, N. J.; Drapkin, B. J. Translesion DNA synthesis mediates acquired resistance to olaparib plus temozolomide in small cell lung cancer. Sci. Adv. 2022, 8, eabn1229.
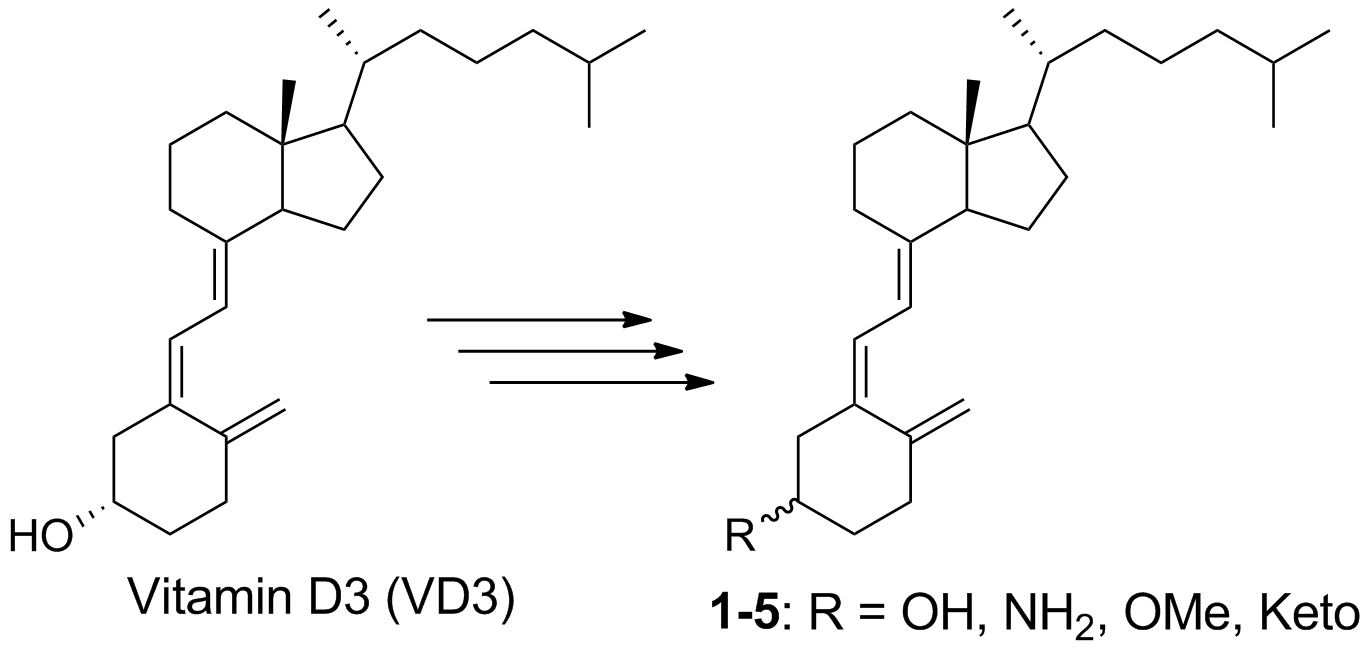
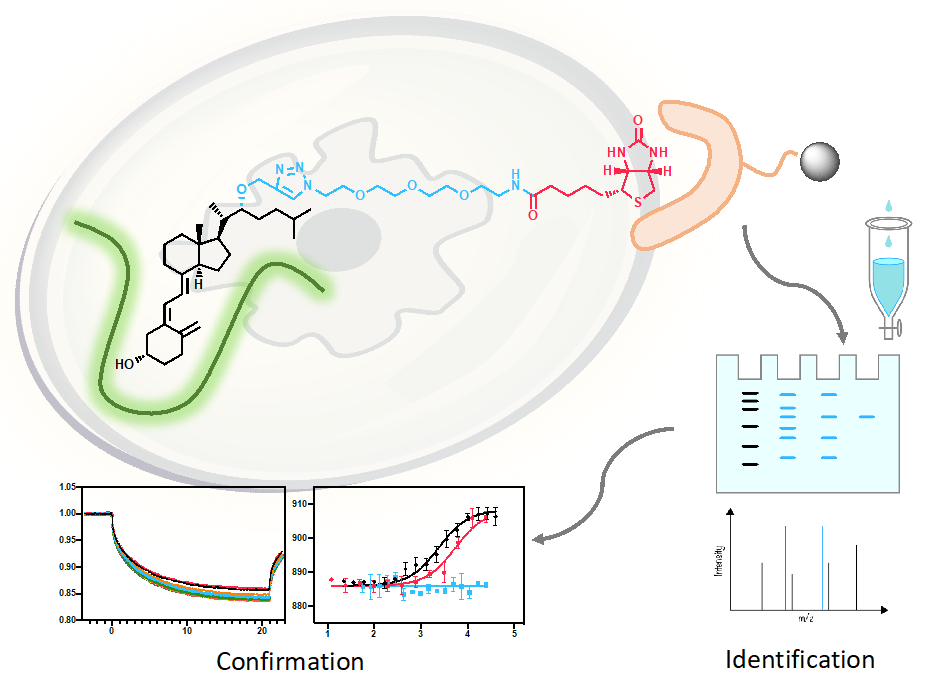
71. Wen, J. Hadden, M. K. Affinity-based protein profiling identifies vitamin D3 as a heat shock protein 70 antagonist that regulates hedgehog transduction in murine basal cell carcinoma. Euro. J. Med. Chem. 2022, 228, 114005.
2021
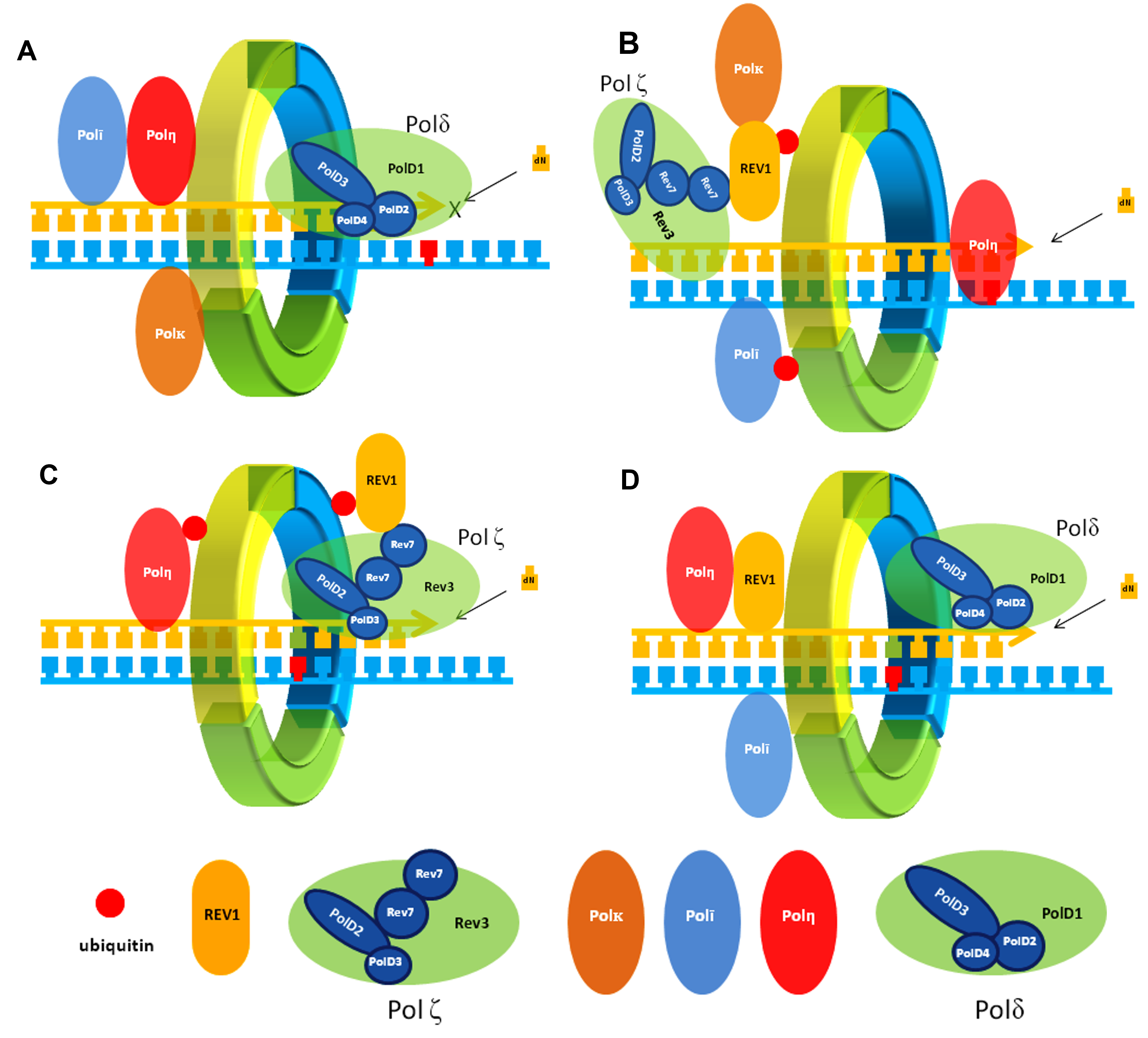
70. Dash, R. C.; Hadden, M. K. Protein-protein interactions in translesion synthesis. Molecules 2021, 26, 5544.
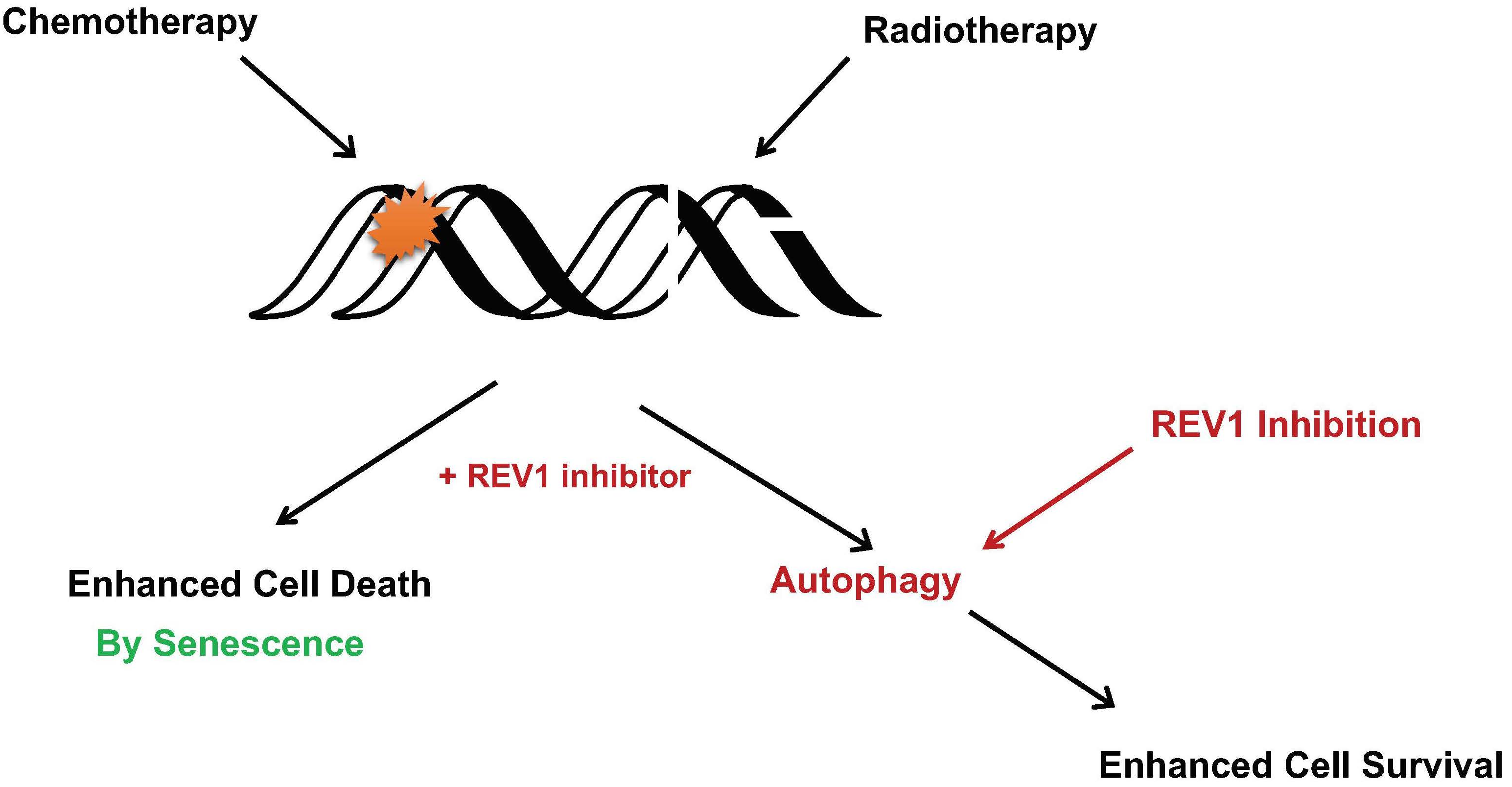
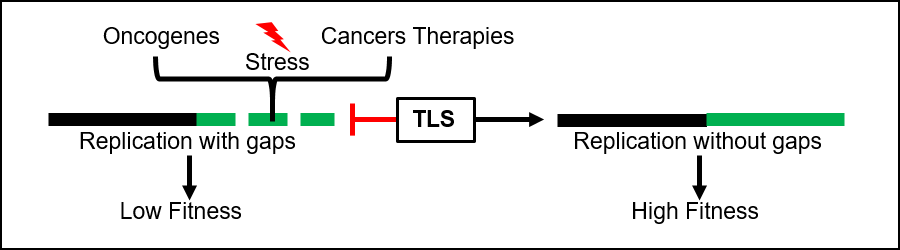
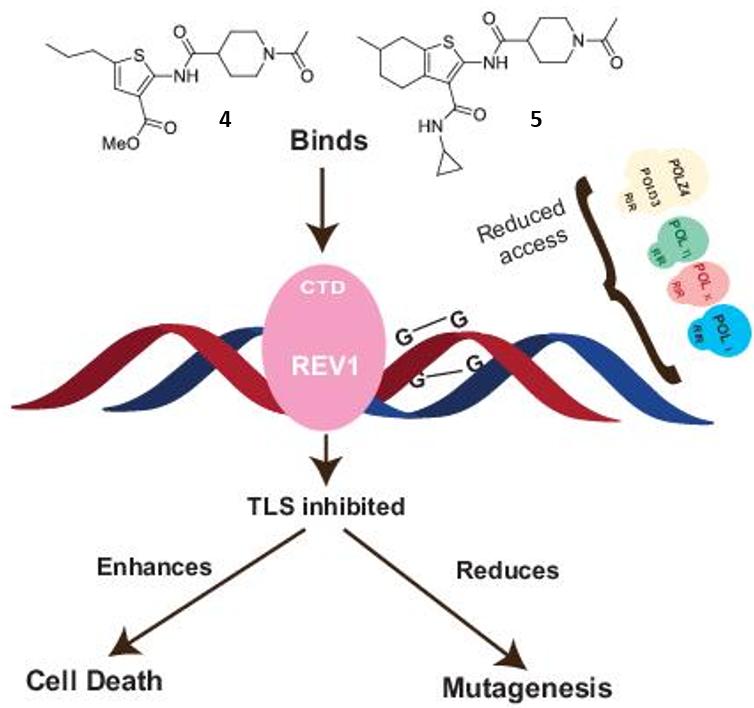
69. Ikeh, K. E.; Lamkin, E. N.; Crompton, A.; Deutsch, J.; Fisher, K. J.; Gray, M.; Argyle, D. J.; Lim, W. Y.; Korzhnev, D. M.; Hadden, M. K.; Hong, J.; Zhou, P.; Chatterjee, N. REV1 inhibition enhances radioresistance and autophagy. Cancers 2021, 13, 5290.
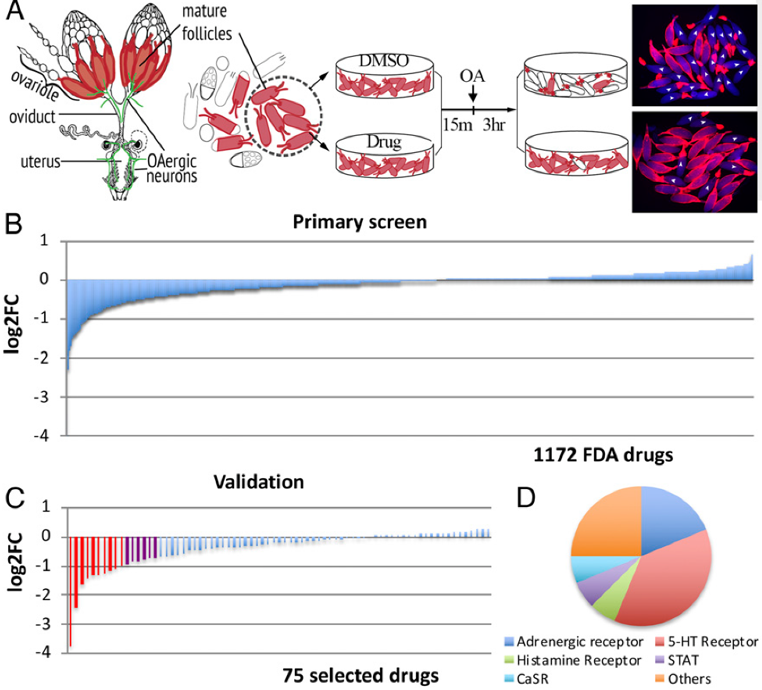
68. Jiang, K.; Zhang, J.;Huang, Y.; Wang, Y.; Xiao, S.; Hadden, M. K.; Woodruff, T. K.; Sun, J. A platform utilizing Drosophila ovulation for nonhormonal contraceptive screening. Proc. Natl Acad. Sci. USA 2021, 118, e2026403118.
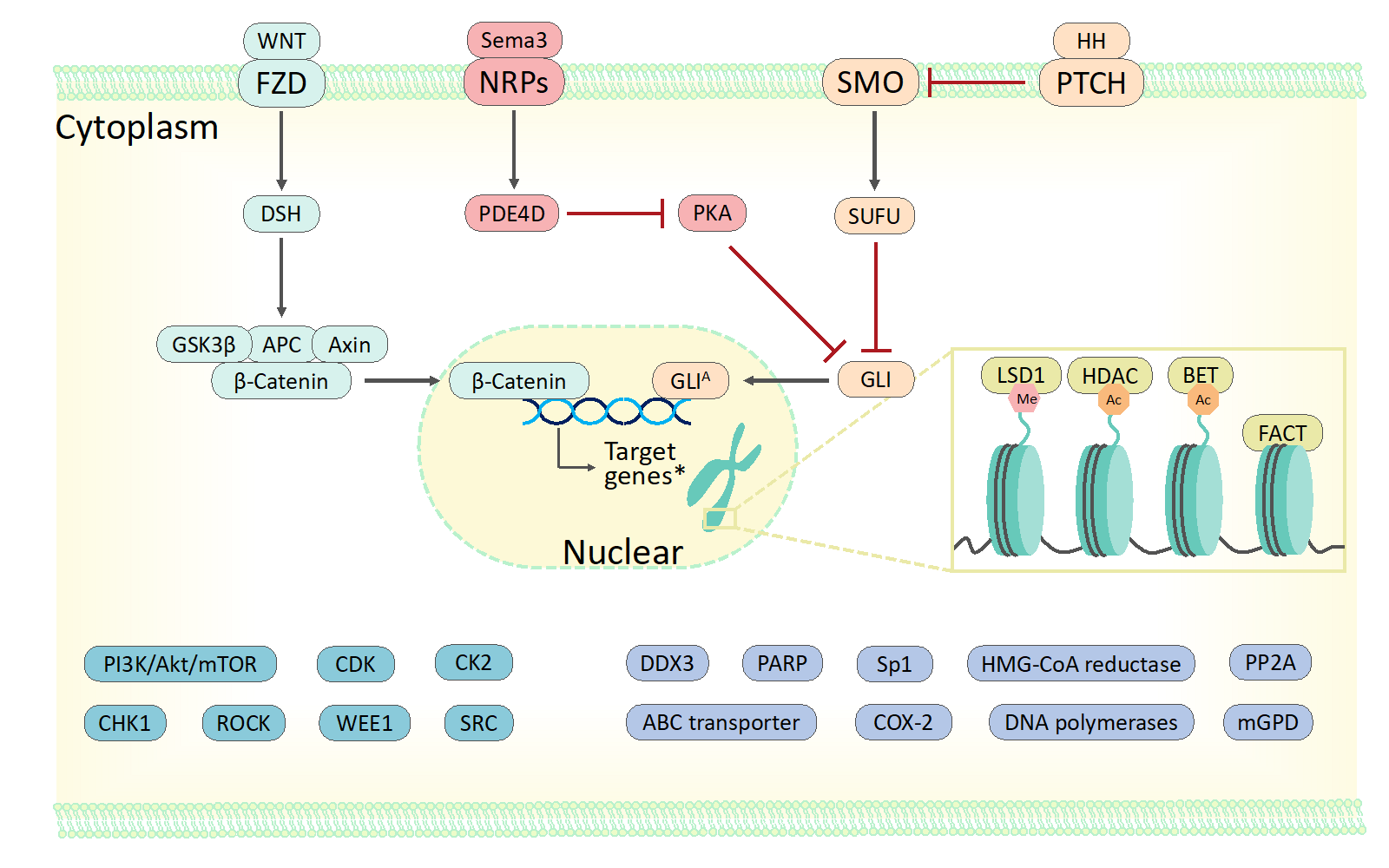
67. Wen, J.; Hadden, M. K. Medulloblastoma drugs in development: Current leads, trials and drawbacks. J. Med. Chem. 2021, 215, 113268.
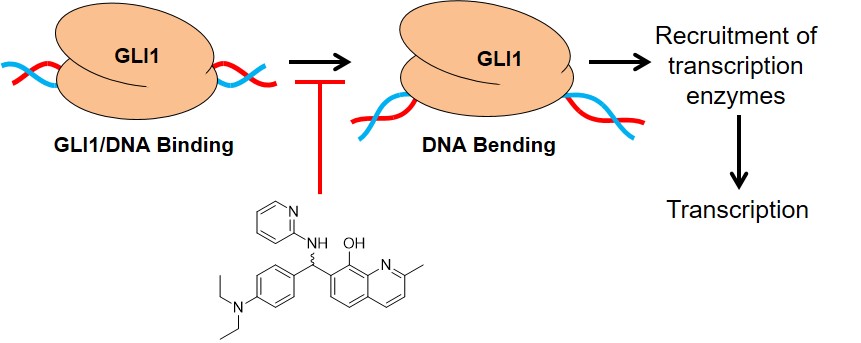
66. Dash, R. C.; Wen, J.; Zaino, A. M.; Morel, S. R.; Chau, L. Q.; Wechsler-Reya, R. J. Hadden, M. K. Structure-based virtual screening identifies an 8-hydroxyquinoline as a small molecule GLI1 inhibitor. Mol. Ther. Oncolytics 2021, 20, 265-276.
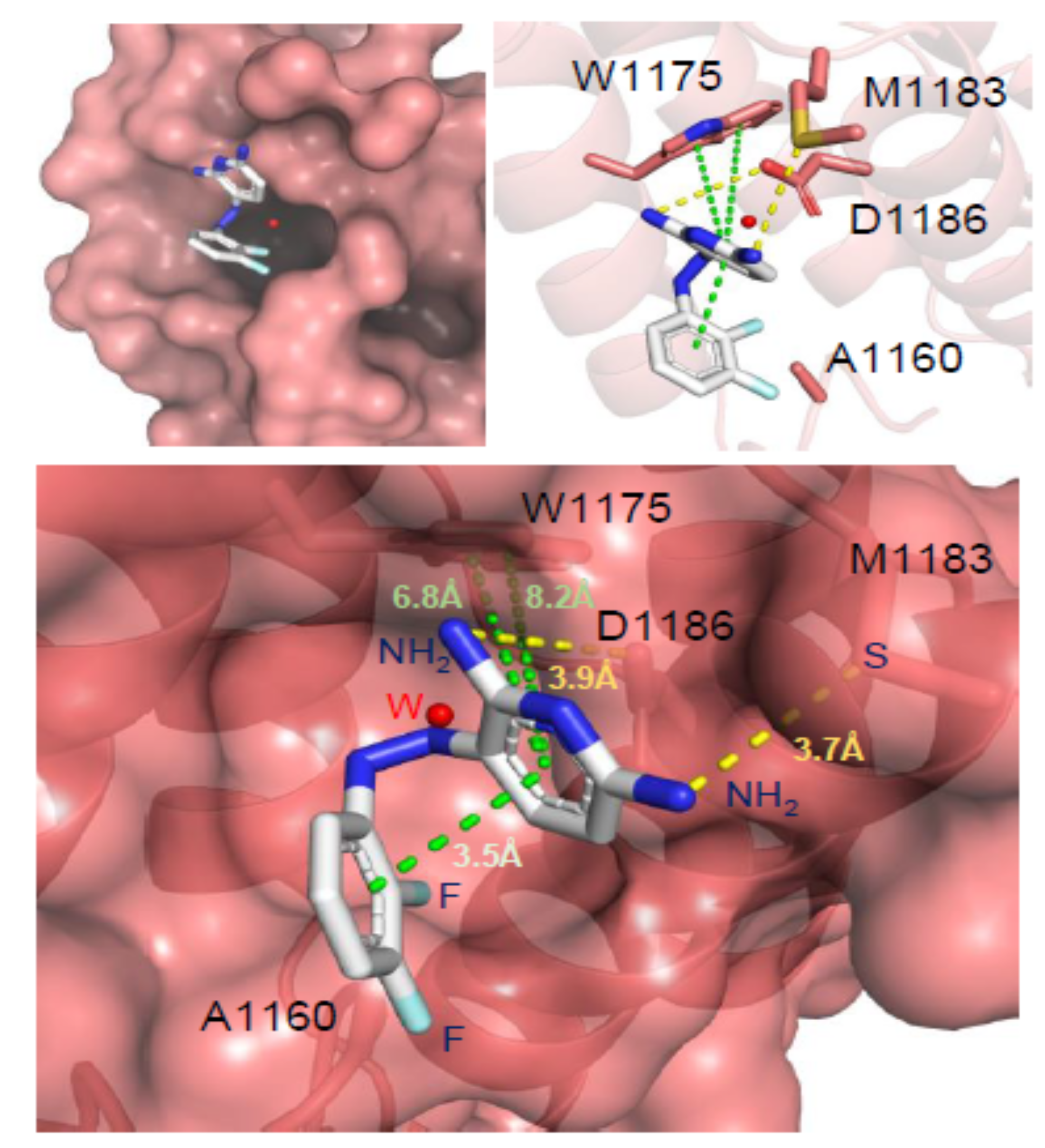
65. McPherson, K. S.; Zaino, A. M.; Dash, R. C.; Rizzo, A. A.; Li, Y.; Hao, B.; Bezsonova, I.; Hadden, M. K.; Korzhnev, D. M. Structure-based drug design of phenazopyridine derivatives as inhibitors of Rev1 interactions in translesion synthesis. ChemMedChem 2021, 16, 1126-1132.
64. Patel, S. M.; Dash, R. C.; Hadden, M. K. Translesion synthesis inhibitors as a new class of cancer chemotherapeutics. Invited Review. Expert Opin. Inv. Drugs. 2021, 30, 13-24.
63. Dusek, C. O.; Hadden, M. K. Targeting the Gli family of transcription factors for the development of anti-cancer drugs. Invited Review. Expert Opin. Drug Discov. 2021, 16, 289-302.
2020
62. Chen, C.; Bosko, C.; McGeough, C.; McLean, R.; Zaino, A. M.; Hadden, M. K.; Peczuh, M. W. Exploring the physicochemical and antiproliferative properties of biaryl-linked [13]-macrodilactones. Bioorg. Med. Chem. 2020, 28, 115671.
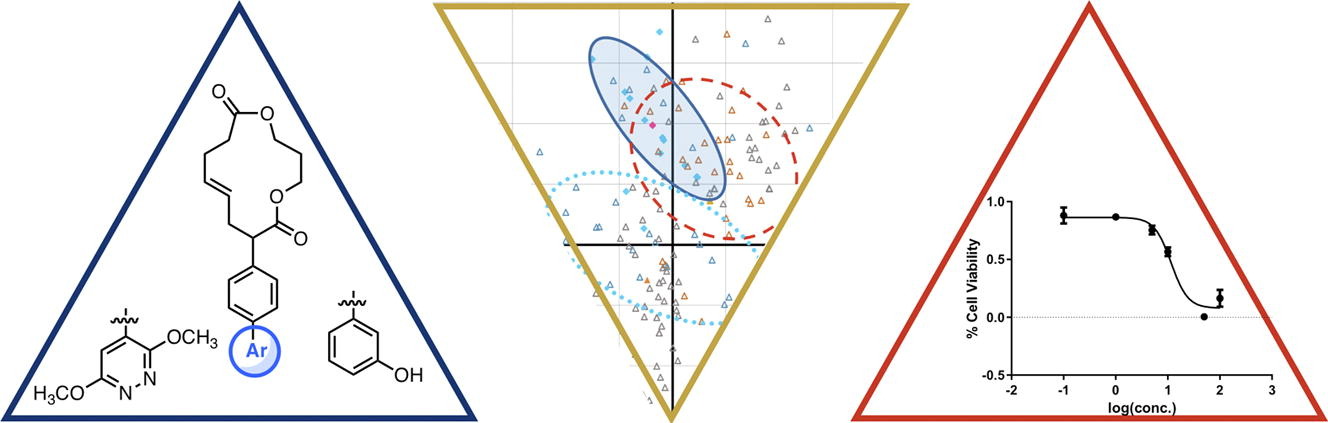
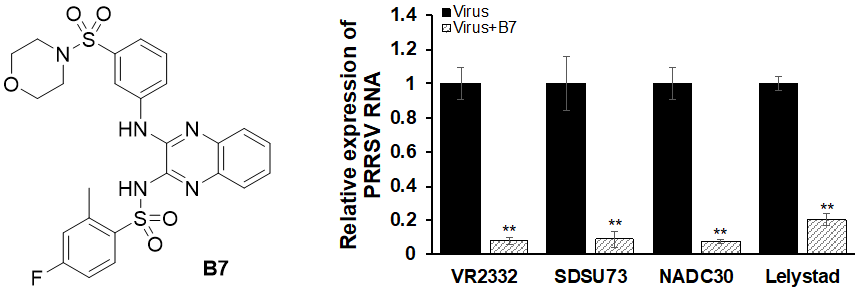
61. Huang, C.; Bernard, D.; Zhu, J.; Dash, R. C.; Chu, A.; Knupp, A.; Hakey, A.; Hadden, M. K.; Garmendia, A.; Tang, Y. Small molecules block the interaction between porcine reproductive and respiratory syndrome virus and CD163 receptor and the infection of pig cells. Virol. J. 2020, 17, 116.
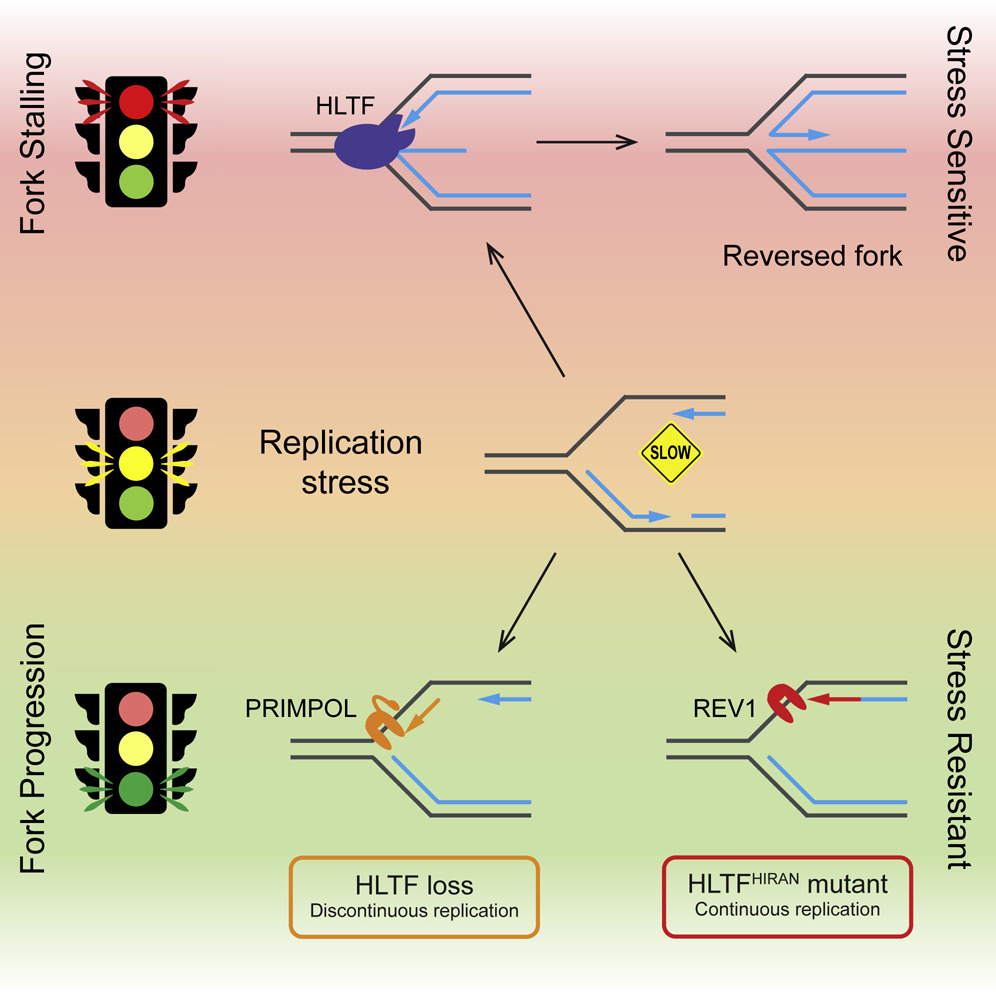
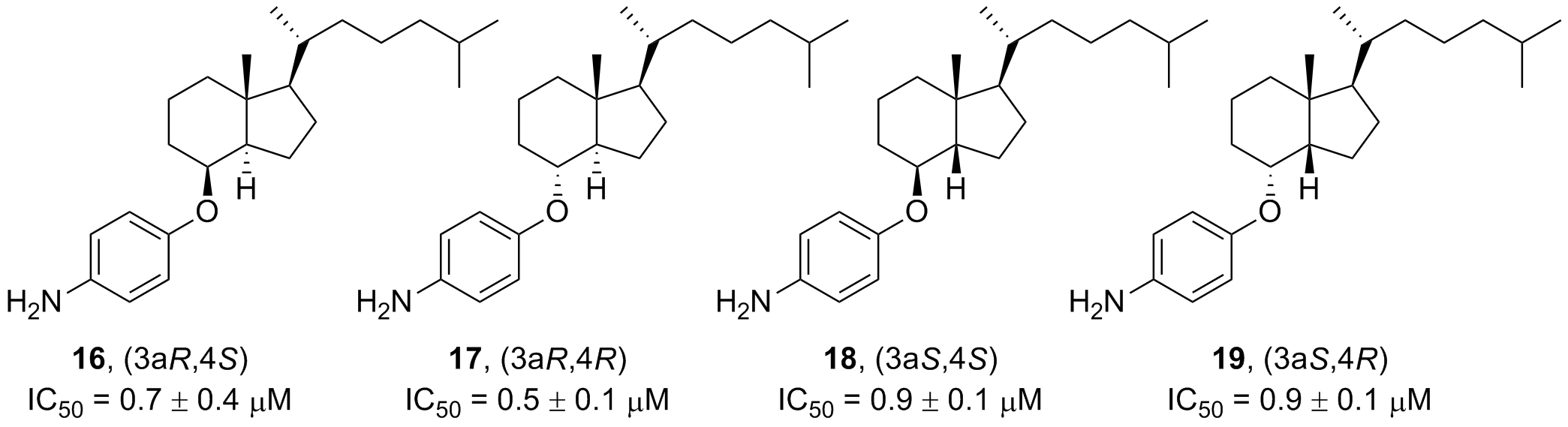
60. Bai, G.; Kermi, C.; Story, H.; Schiltz, C.; Bacal, J.; Zaino, A. M.; Hadden, M. K.; Eichman, B.; Lopes, M.; Cimprich, K. A. HLTF promotes fork reversal, limiting replication stress resistance and preventing multiple mechanisms of unrestrained DNA synthesis. Mol. Cell 2020, 78, 1237-1251.
59. Hadden, M. K.; Zaino, A. M. Introduction to medicinal chemistry: A five-day course for high school students. J. Chem. Ed. 2020, 97, 1543-1548.
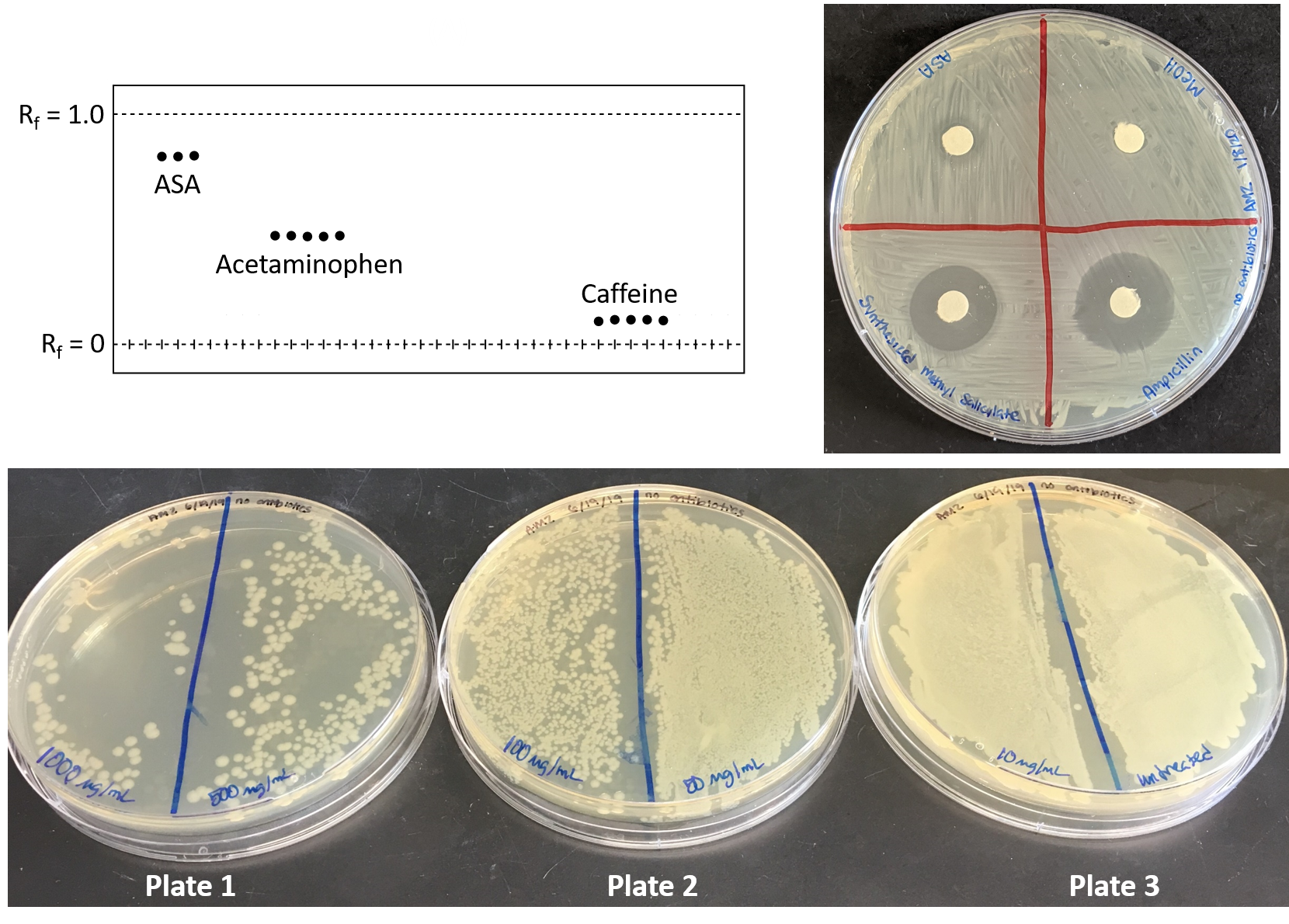
58. Nayak, S.; Calvo, J. A.; Cong, K.; Peng, M.; Berthiaume, E.; Jackson, J.; Zaino, A. M.; Vindigni, A.; Hadden, M. K.; Cantor, S. B. Inhibition of the translesion synthesis polymerase Rev1 exploits replication gaps as a cancer vulnerability. Sci. Adv. 2020, 6, eaaz7808.
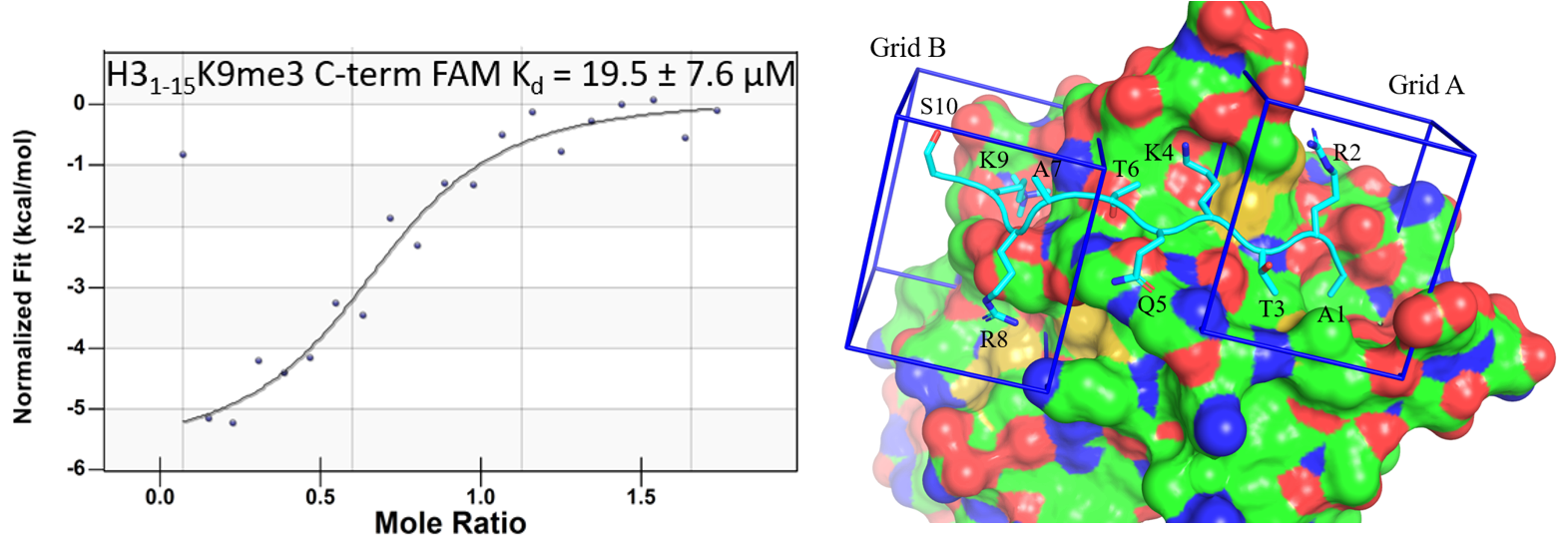
57. Zaino, A. M.; Dash, R. C.; Hadden, M. K. Probing the protein-protein interaction between the ATRXADD domain and the Histone H3 tail. Molecules 2020, 25, 1500.
56. Wen, J.; Teske, K. A.; Hadden, M. K. Inhibition of hedgehog signaling by stereochemically defined des-triazole itraconazole analogues. Bioorg. Med. Chem. Lett. 2020, 30, 126794.
55. Pace, J. R. ; Jog, R.; Burgess, D. J.; Hadden, M. K. Formulation and evaluation of itraconazole liposomes for hedgehog pathway inhibition. J. Liposome Res. 2020, 30, 305-311.
2019
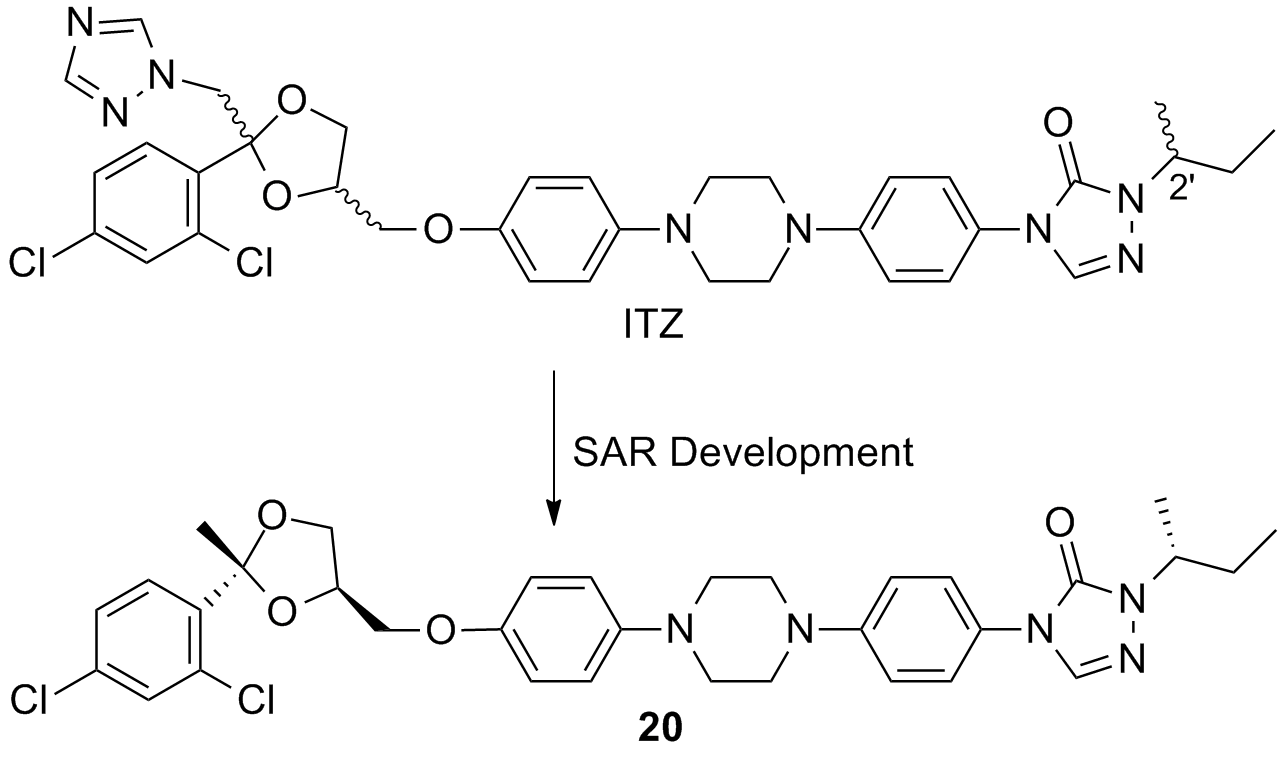
54. Wen, J. Chennamadhavuni, D.; Morel, S. R.; Hadden, M. K. Truncated itraconazole analogues exhibiting potent anti-hedgehog activity and improved drug-like properties. ACS Med. Chem. Lett. 2019, 10, 1290-1295.
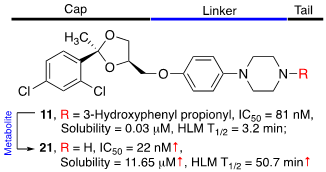
53. Dash, R. C.; Ozen, Z.; McCarthy, K. R.; Chatterjee, N.; Harris, C. A.; Rizzo, A. A.; Walker, G. C.; Lorzhnev, D. M.; Hadden, M. K. Virtual pharmacophore screening identifies small-molecule inhibitors of the Rev1-CT/RIR protein-protein interaction. ChemMedChem 2019, 14, 1610-1617.
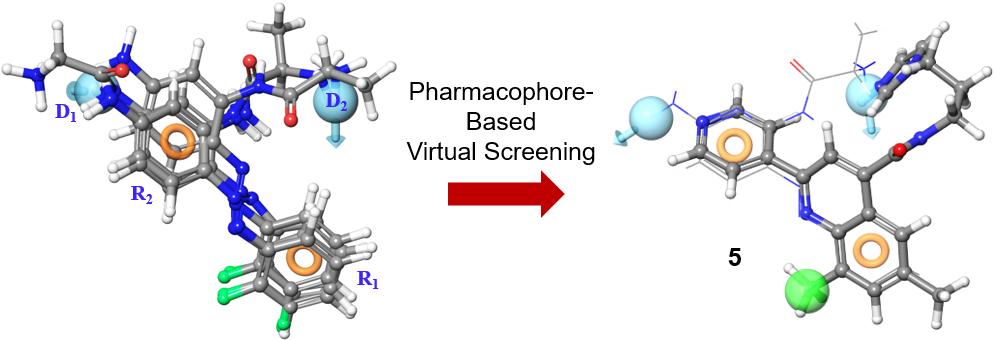
52. Pace, J. R.; Teske, K. A.; Chau, L. Q.; Dash, R. C.; Zaino, A. M.; Wechsler-Reya, R. J.; Hadden, M. K. Structure-activity relationships for itraconazole-based triazolone analogues as hedgehog pathway inhibitors. J. Med. Chem. 2019, 62, 3873-3885.
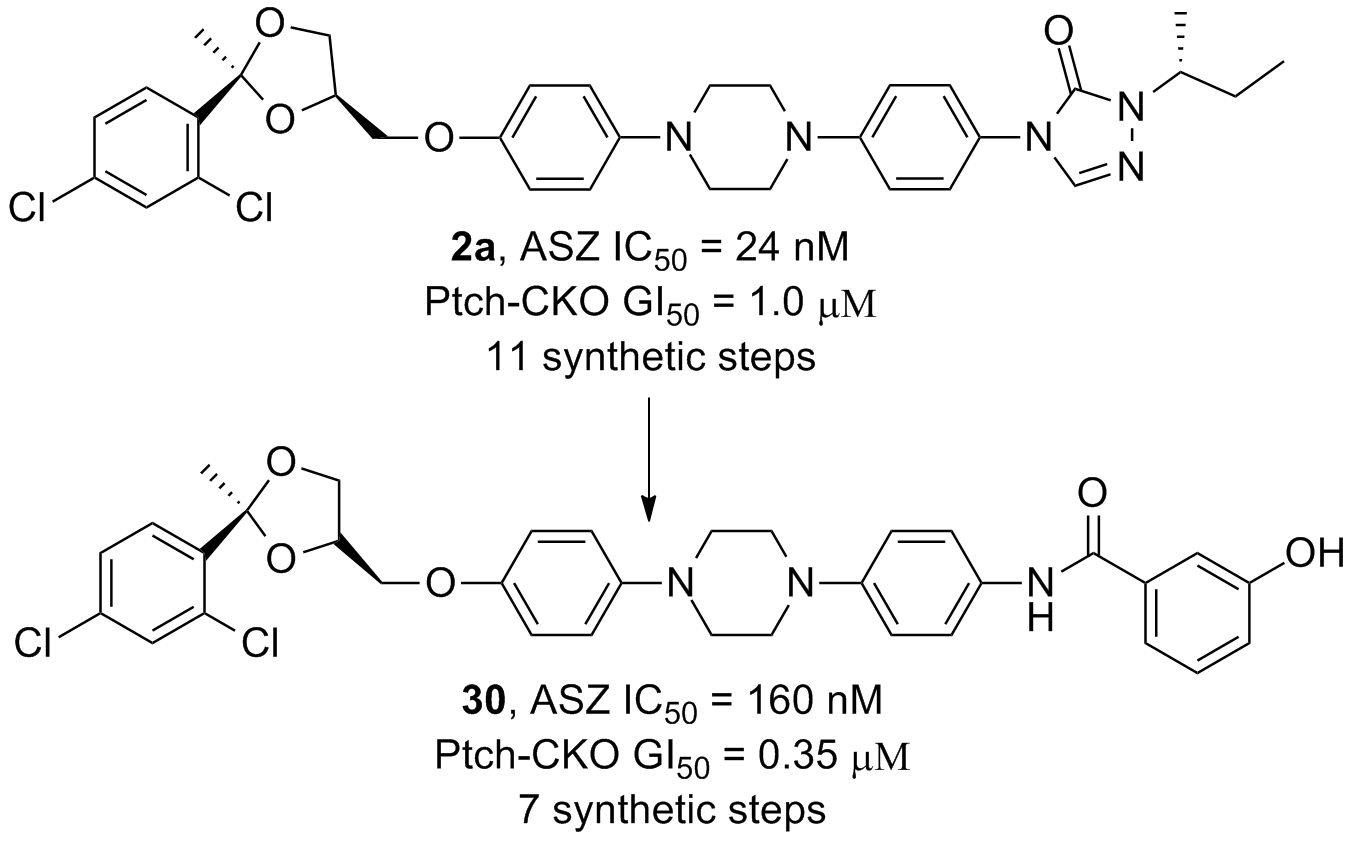
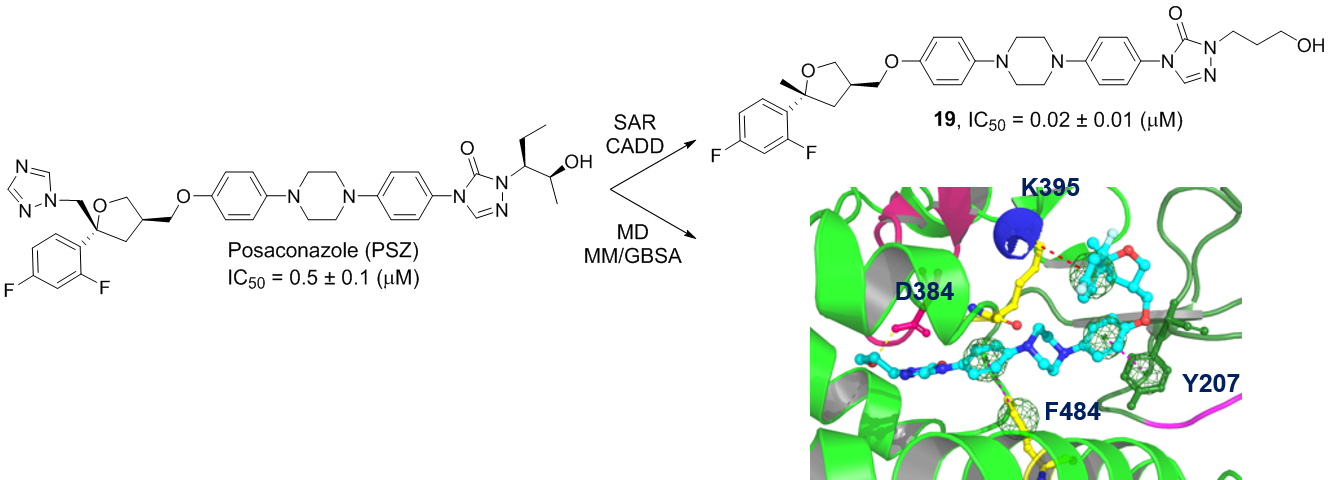
51. Teske, K. A.; Dash, R. C.; Morel, S. R.; Chau, L. Q.; Wechsler-Reya, R. J.; Hadden, M. K. Development of posaconazole-based analogues as hedgehog signaling pathway inhibitors. Euro. J. Med. Chem. 2019, 163, 320-332.
50. Maschinot C. A.; Chau, L. Q.; Wechsler-Reya, R. J.; Hadden, M. K. Synthesis and evaluation of third generation vitamin D3 analogues as inhibitors of hedgehog signaling. Euro. J. Med. Chem. 2019, 162, 495-506.
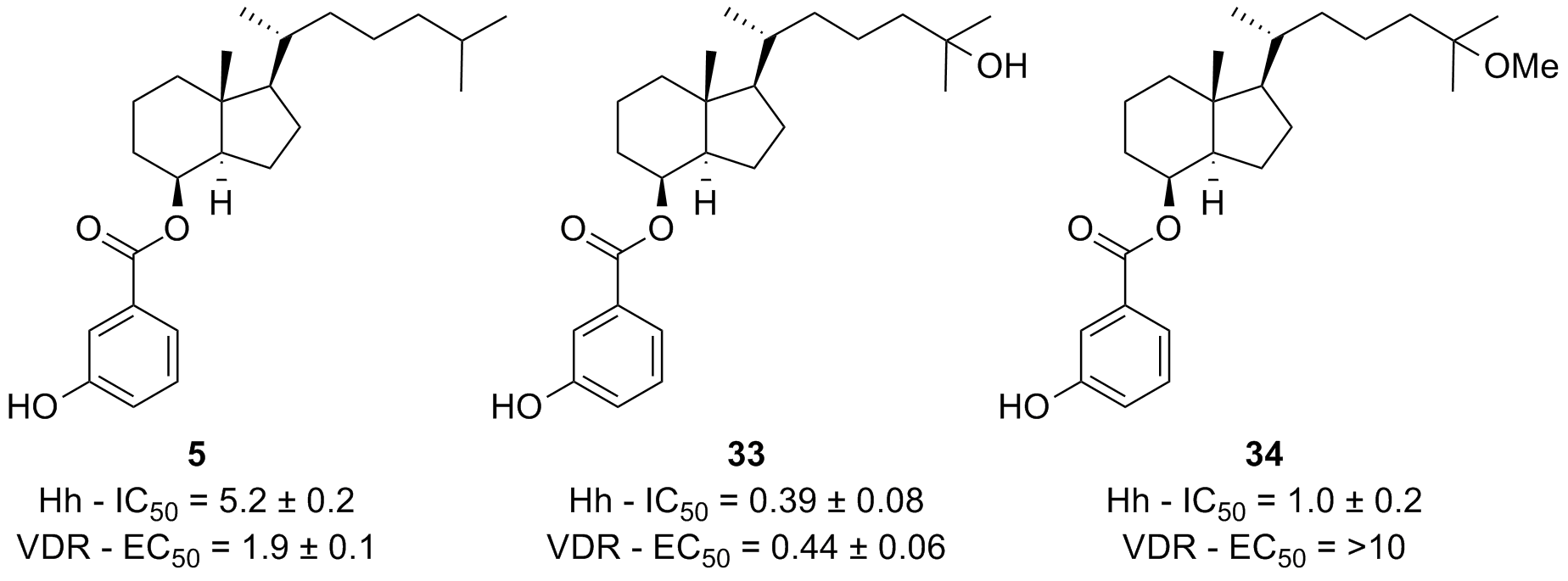
49. Thompson, E. N.; Sail, V.; Raccuia, D. S.; Hadden, M. K. Probing seco-steroid inhibition of the hedgehog signaling pathway. Mol. Cell. Biochem. 2019, 450, 75-85.
2018
48. Dash, R. C.; Ozen, Z.; Rizzo, A. A.; Lim, S.; Korzhnev, D. M.; Hadden, M. K. Structural approach to identify a lead scaffold that targets the translesion synthesis polymerase Rev1. J. Chem. Inf. Model. 2018, 58, 2266-2277.
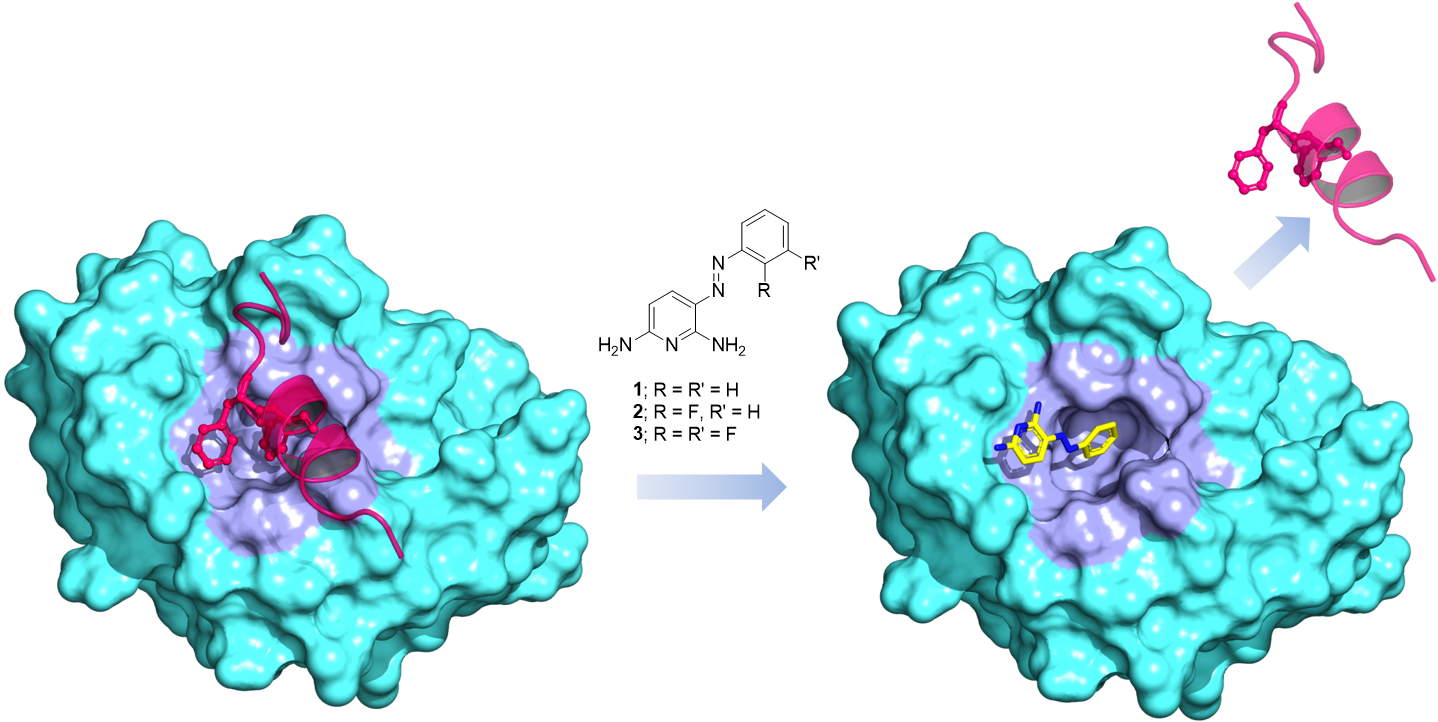
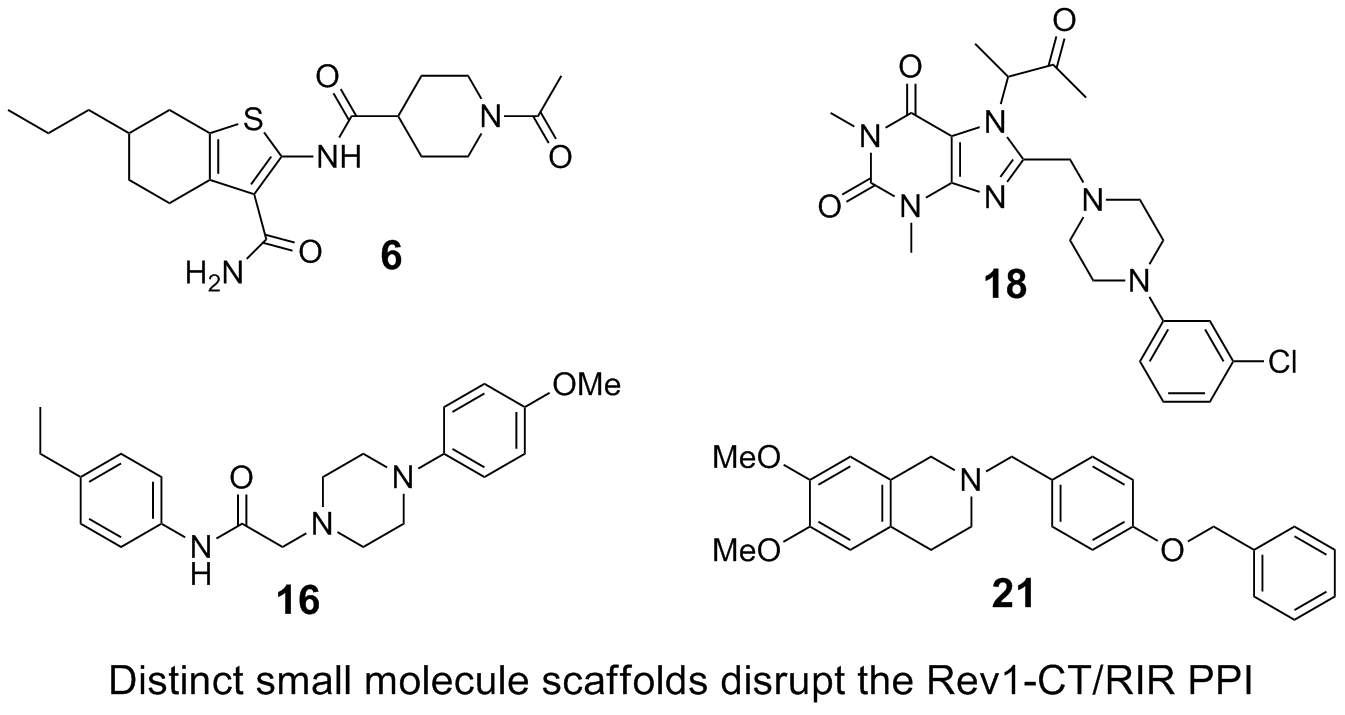
47. Ozen, V.; Dash, R. C.; McCarthy, K. R.; Chow, S. A.; Rizzo, A. A.; Korzhnev, D. M.; Hadden, M. K. Small molecule scaffolds that disrupt the Rev1-CT/RIR protein-protein interaction. Bioorg. Med. Chem. 2018, 26, 4301-4309.
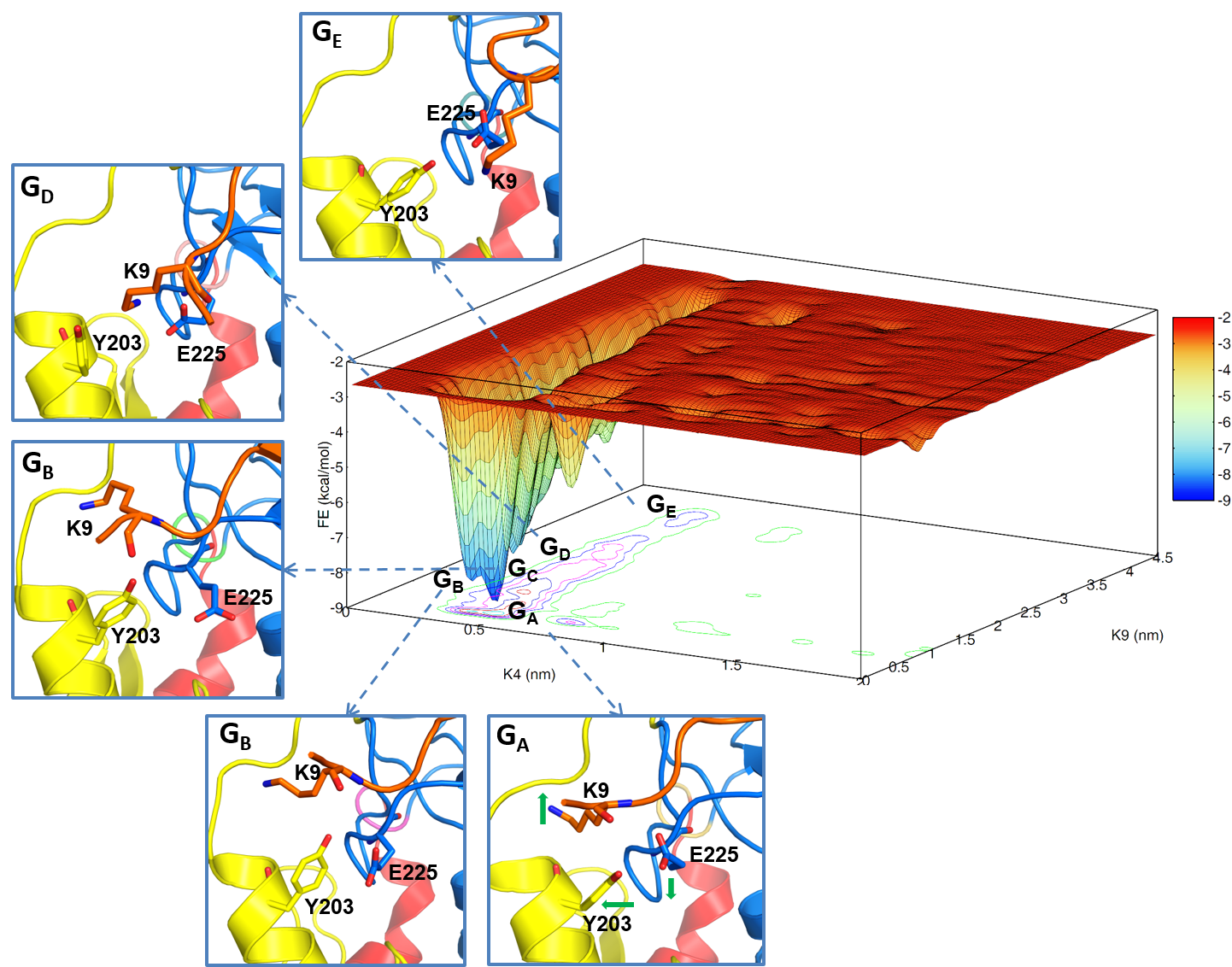
46. Dash, R. C.; Zaino, A. M.; Hadden, M. K. A Metadynamic Approach to Understand the Recognition Mechanism of the Histone H3 Tail with the ATRXADD Domain. BBA Gene Regul. Mech. 2018, 1861, 594-602.
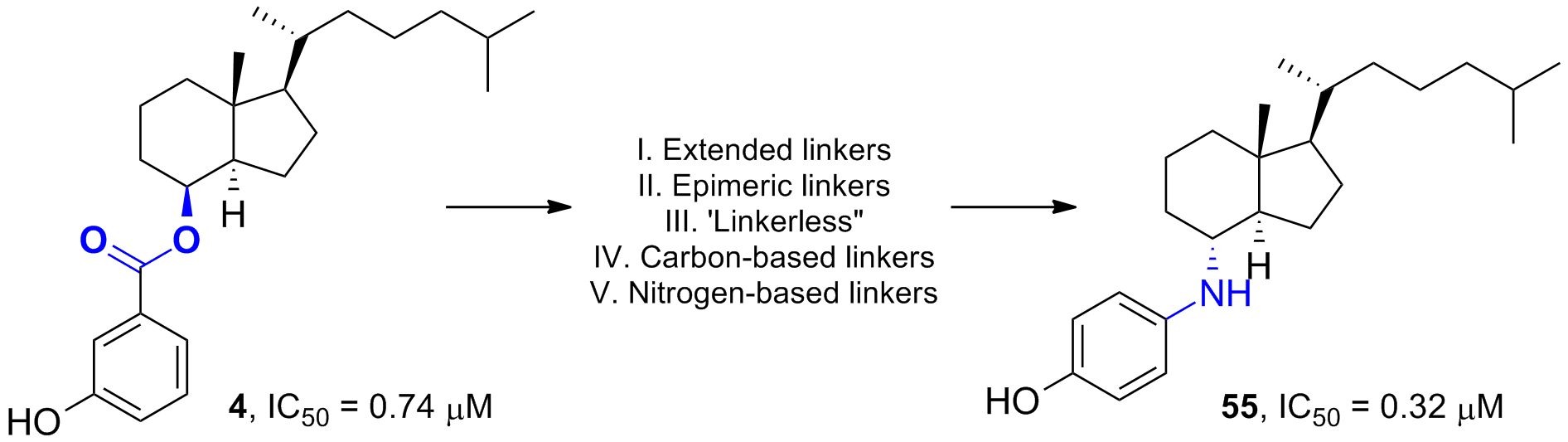
45. Wen, J. and Hadden, M. K. Structure–Activity Relationship Studies of Vitamin D3 Analogues Containing an Ether or Thioether Linker as Hedgehog Pathway Inhibitors. ChemMedChem 2018, 13, 748-753.
2017
44. Maschinot, C. A. and Hadden, M. K. Synthesis and evaluation of vitamin D3 analogus with C-11 modifications as inhibitors of hedgehog signaling. Bioorg. Med. Chem. Lett. 2017, 27, 4011-4014.
43. Sail, V.; Rizzo, A. A.; Chatterjee, N.; Dash, R. C.; Ozen, Z.; Walker, G. C.; Korzhnev, D. M.; Hadden, M. K. Identification of small molecule translesion synthesis inhibitors that target the Rev1-CT/RIR protein-protein interaction. ACS Chem. Biol. 2017, 12, 1903-1912.
42. Dash, R. C.; Maschinot, C. M.; Hadden, M. K. A molecular dynamics approach to identify an oxysterol-based hedgehog pathway inhibitor. Biochim. Biophys. Acta 2017, 1861, 168-177.

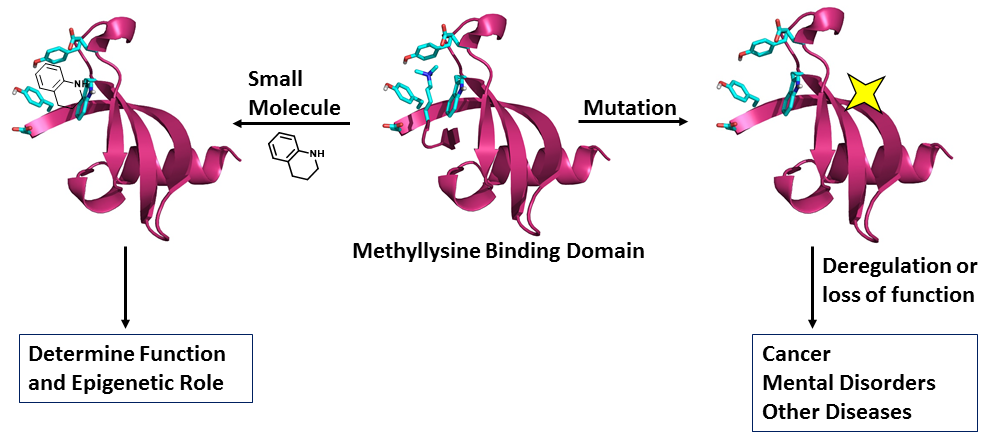
41. Teske, K. A. and Hadden, M. K. Methyllysine binding domains: Structural insight and small molecule probe development. Euro. J. Med. Chem. 2017, 136, 14-35.
2016
40. Pace, J. R.; DeBerardinis, A. M.; Sail, V.; Tacheva-Grigorova, S. K.; Chan, K. A.; Tran, R.; Raccuia, D. S.; Wechsler-Reya, R.; Hadden, M. K. Repurposing the clinically efficacious anti-fungal agent itraconazole as an anti-cancer chemotherapeutic. J. Med. Chem. 2016, 59, 3635-3649.
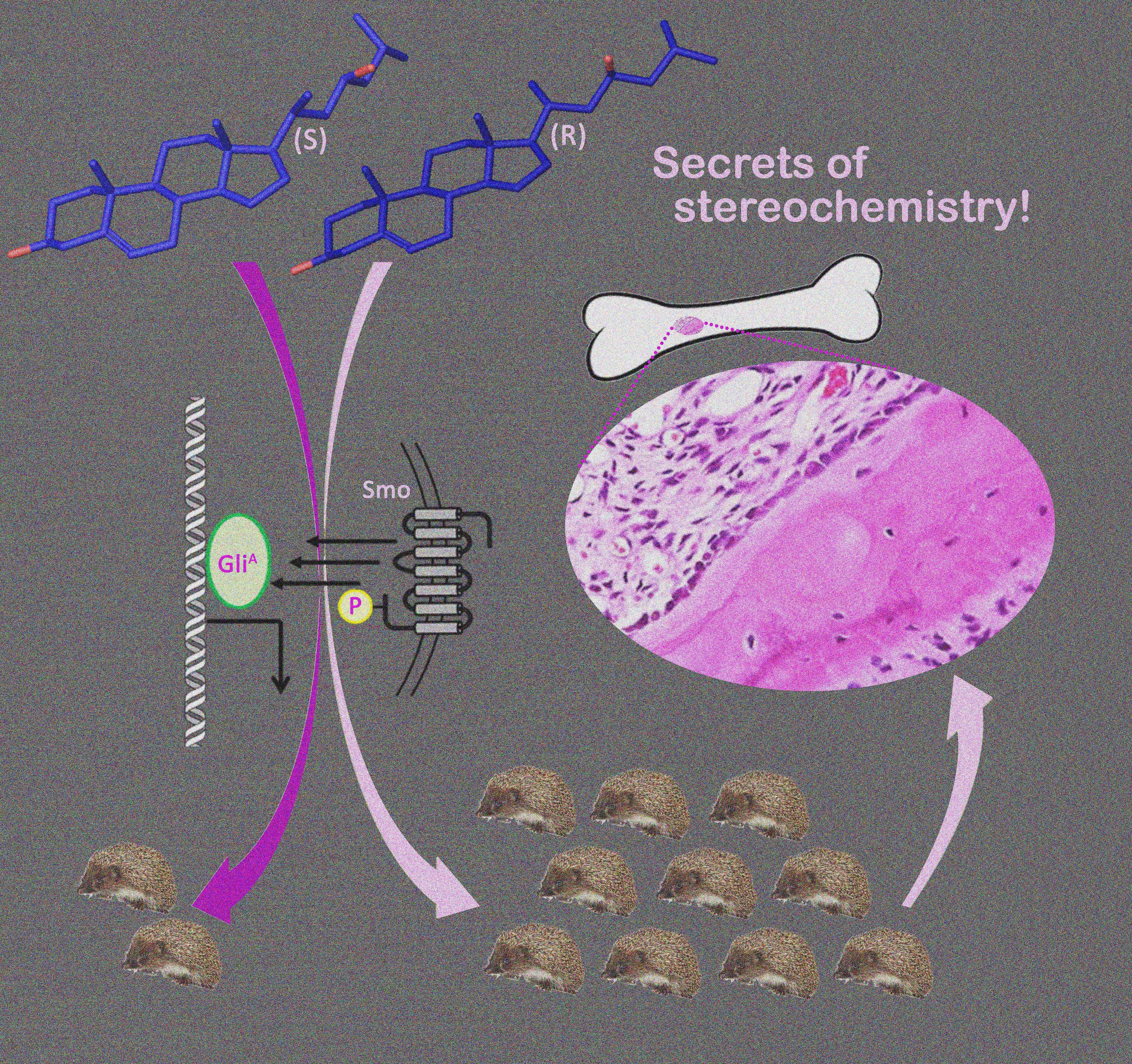
***39. Maschinot, C. A.; Corman, A. R.; DeBerardinis, A. M.; Hadden, M. K. Synthesis and evaluation of osteogenic oxysterols as hedgehog pathway activators. ChemMedChem 2016, 11, 679-686.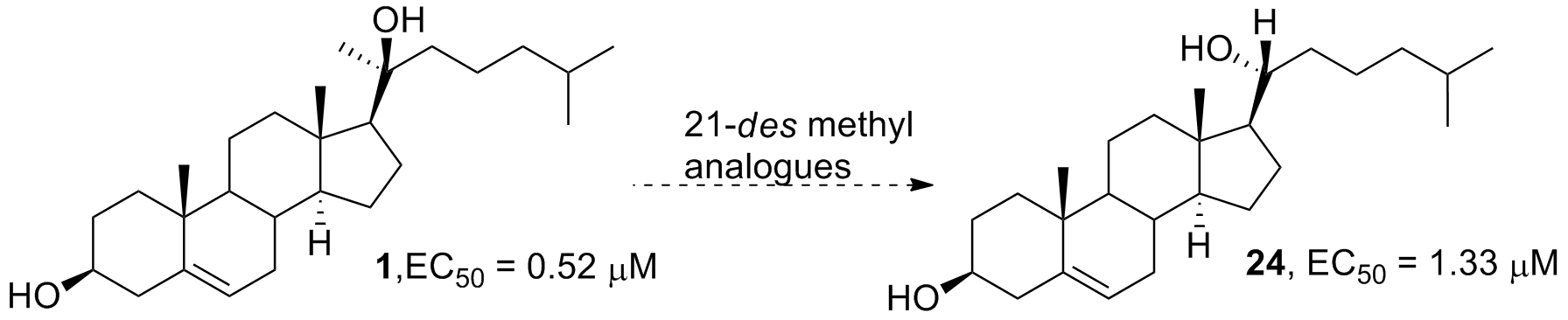
***Featured article – “The front cover picture shows how the stereochemical orientation of the C-21 methyl group in the oxysterol (OHC) scaffold can determine whether the OHC serves as an agonist of the hedgehog (Hh) signaling pathway (hedgehog image by Marina Maslennikova, iStockphoto). The natural configuration of the C-21 methyl is R, which is shown here in the structure of 23(S)-hydroxycholesterol (4, right), a potent, synthetic agonist of Hh signaling with the ability to promote osteogenic differentiation. Inversion of the C-21 methyl to the S configuration (16, left) completely abolished its ability to activate Hh signaling. These results provide essential structural insight for the future development of small molecule OHCs as Hh pathway agonists.”
38. Korzhnev, D. M. and Hadden, M. K. Targeting the translesion synthesis pathway for the development of anti-cancer chemotherapeutics. J. Med. Chem. 2016, 59, 9321-9336.
2015
37. DeBerardinis, A. M.; Raccuia, D. S.; Maschinot, C. A.; Thompson, E.; Hadden, M. K. Vitamin D3 analogues that contain modified A- and seco-B-rings as hedgehog pathway inhibitors. Euro. J. Med. Chem. 2015, 93, 156-171.
36. Banerjee, U.; DeBerardinis, A. M.; Hadden, M. K. Design, synthesis, and evaluation of hybrid vitamin D3 side chain analogues as hedgehog pathway inhibitors. Bioorg. Med. Chem. 2015, 23, 548-555.
35. Maschinot, C. A.; Pace, J.; Hadden, M. K. Synthetic small molecule inhibitors of Hh signaling as anti-cancer chemotherapeutics. Curr. Med. Chem. 2015, 22, 4033-4057.
34. Hadden, M. K. Targeting GLI proteins in human cancer by small molecules (WO2014116651 A1): a patent evaluation. Exp. Opin. Ther. Patents 2015, 25(5).
2014
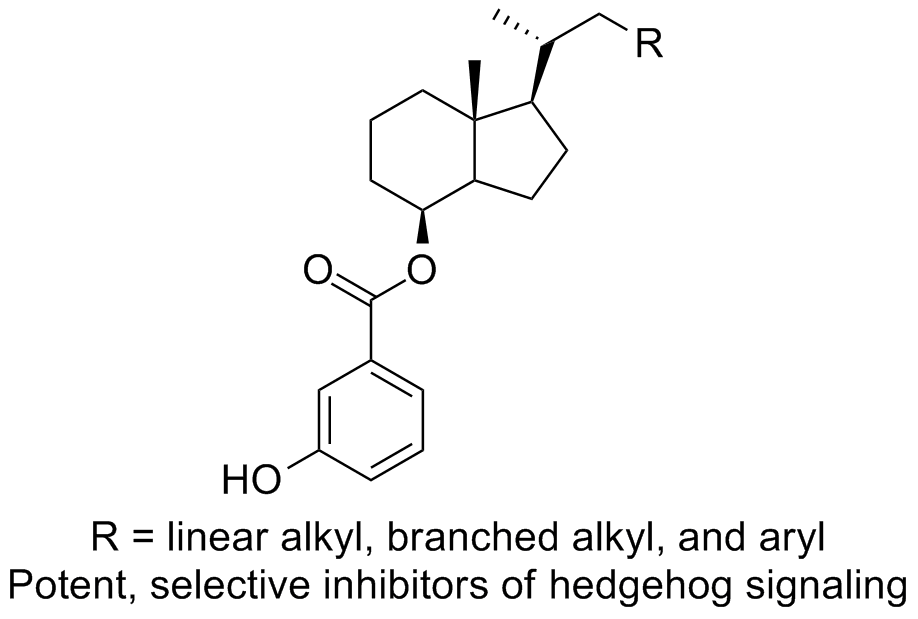
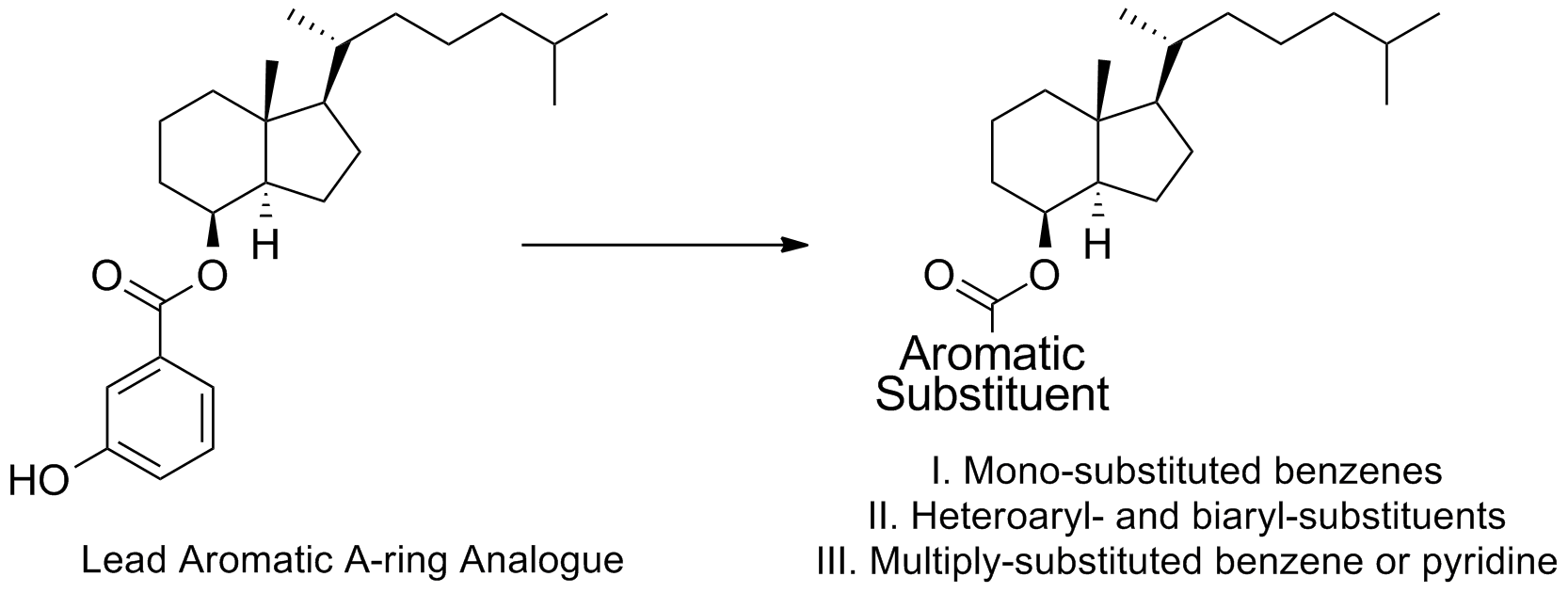
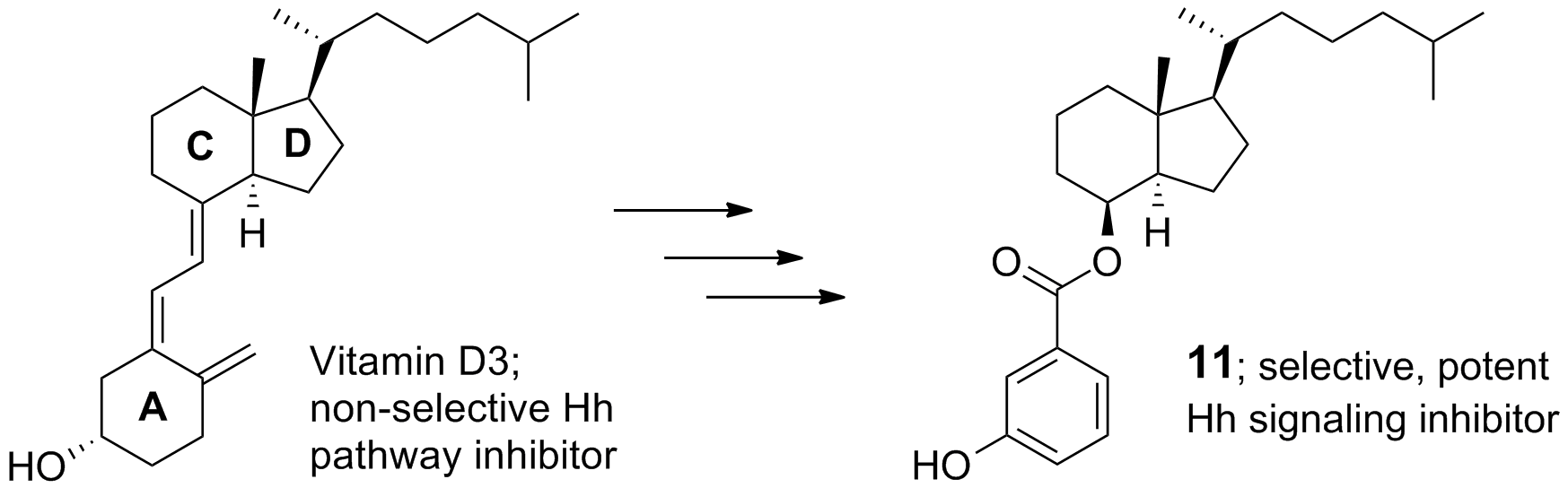
33. DeBerardinis, A. M.; Madden, D. J.; Banerjee, U.; Sail, V.; Raccuia, D. S.; De Carlo, D.; Lemieux, S. M.; Meares, A.; Hadden, M. K. Structure-activity relationships for vitamin D3-based aromatic A-ring analogues as hedgehog pathway inhibitors. J. Med. Chem. 2014, 57, 3724-3736.
32. Banerjee, U.; Hadden, M. K. Recent advances in the design of hedgehog pathway inhibitors for the treatment of malignancies. Exp. Opin. Drug Discov. 2014, 9, 751-771.
31. Hadden, M. K. Hedgehog pathway agonism: Therapeutic potential and small molecule development. ChemMedChem 2014, 9, 27-37.
2013
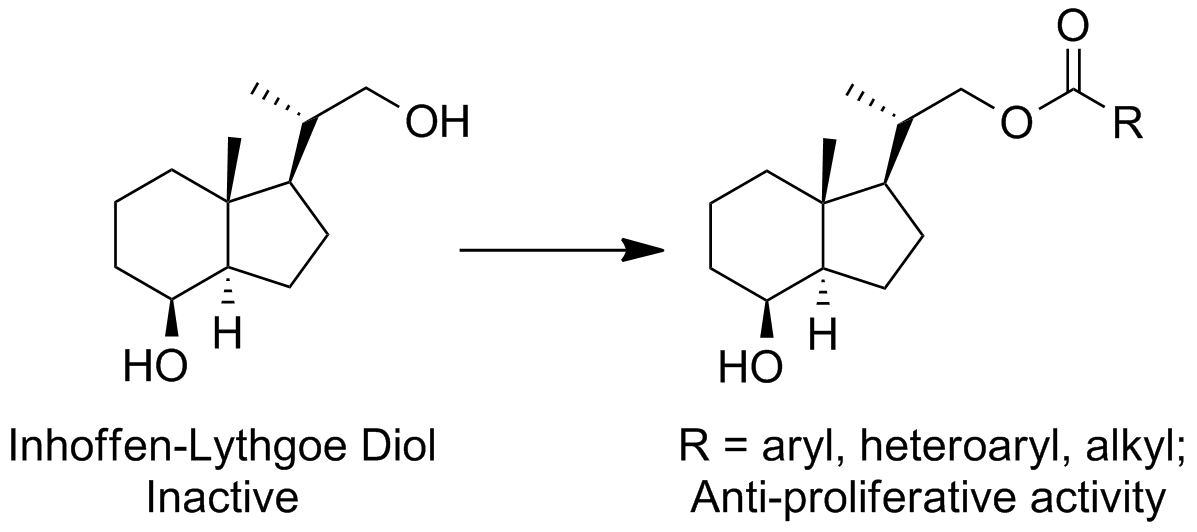
30. DeBerardinis, A. M.; Lemieux, S. M.; Hadden, M. K. Analogues of the Inhoffen-Lythgoe diol with anti-proliferative activity. Bioorg. Med. Chem. Lett. 2013, 23, 5367-5370.
29. DeBerardinis, A. M.; Banerjee, U.; Hadden, M. K. Identification of vitamin D3-based hedgehog pathway inhibitors that incorporate an aromatic A-ring isostere. ACS Med. Chem. Lett. 2013, 4, 590-595.
28. Sail, V.; Hadden, M. K. Identification of small molecule Hes1 modulators as potential anti-cancer chemotherapeutics. Chem. Biol. & Drug Design 2013, 81, 334-342.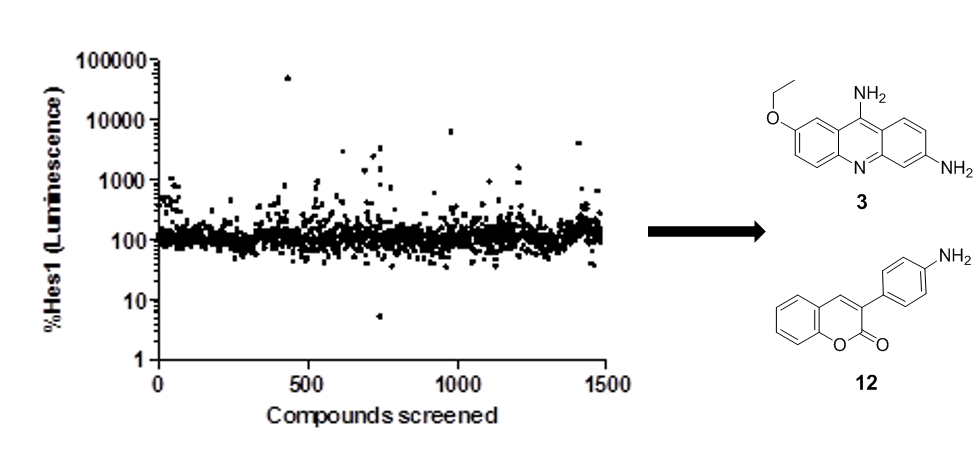
27. Lemieux, S.; Hadden, M. K. Targeting the fibroblast growth factor receptors for the treatment of cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 748-761.
26. Hadden, M. K. Hedgehog pathway inhibitors: a patent review (2009-present). Exp. Opin. Ther. Patents 2013, 23, 345-361.
2012
25. Oblak, E. Z.; Bolstad, E. S. D.; Ononye, S. N.; Priestley, N. D.; Hadden, M. K.; Wright, D. L. The furan route to tropolones: probing the antiprolierative effects of β-thujaplicin analogs. Org. Biomol. Chem. 2012, 10, 8597-8604.
24. Viswanathan, K.; Ononye, S. N.; Cooper, H. D.; Hadden, M. K.; Anderson, A. C.; Wright, D. L. Viridin analogs derived from steroidal building blocks. Bioorg. Med. Chem. Lett. 2012, 22, 6919-6922.
23. Corman, A.; DeBerardinis, A.; Hadden, M. K. Structure-activity relationships for side chain oxysterol agonists of hedgehog signaling. ACS Med. Chem. Lett. 2012, 3, 828-833.
22. DeBerardinis, A.; Banerjee, U.; Miller, M.; Lemieux, S.; Hadden, M. K. Probing the structural requirements for vitamin D3 inhibition of hedgehog signaling. Bioorg. Med. Chem. Lett. 2012, 22, 4859-4863.
21. Banerjee, U.; Ghosh, M.; Hadden, M. K. Evaluation of vitamin D3 A-ring analogues as hedgehog pathway inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1330-1334.
20. Sail, V.; Hadden, M. K. Notch pathway modulators as anti-cancer chemotherapeutics. Ann. Rep. Med. Chem. Ed. Manoj Desai. Vol. 47, ARMC, UK: Academic Press, 2012, 267-280.
19. DeCarlo, D.; Hadden, M. K. Oncoepigenomics: Making histone-lysine methylation count. Euro. J. Med. Chem. 2012, 56, 179-194.
Pre-2010
18. Blagg, B. S. J.; Hadden, M. K. “Hsp90 Inhibitors.” Burger’s Medicinal Chemistry, Drug Discovery and Development. Eds. Donald Abraham and David Rotella, 7th ed. Vol 6. 2010.
17. Hadden, M. K.; Blagg, B. S. J. Synthesis and evaluation of radamide analogues, a chimera of radicicol and geldanamycin. J. Org. Chem. 2009, 74, 4697-4704.
16. Hadden, M. K.; Hill, S.; Davenport, J.; Matts, R. L.; Blagg, B. S. J. Synthesis and evaluation of Hsp90 inhibitors that contain the 1,4-naphthoquinone scaffold. Bioorg. Med. Chem. 2009, 17, 634-640.
15. Hadden, M. K.; Blagg, B. S. J. Dimeric approaches to anti-cancer chemotherapeutics. Anti-Cancer Agents Med. Chem. 2008, 8, 807-816.
14. Tash, J. S.; Chakrasali, R.; Jakkaraj, S. R.; Hughes, J.; Smith, S. K.; Hornbaker, K.; Heckert, L. L.; Ozturk, S. B.; Hadden, M. K.; Kinzy, T. G.; Blagg, B. S. J.; Georg, G. I. Gamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP-90, eEF1A, and stimulates IL-1 transcription in Sertoli cells. Biol. Reprod. 2008, 78, 1139-1152.
13. Hastings, J. M.; Hadden, M. K.; Blagg, B. S. J. Synthesis and evaluation of derrubone and select analogues. J. Org. Chem. 2008, 73, 369-373.
12. Hadden, M. K.; Galam, L.; Gestwicki, J. E.; Matts, R. L.; Blagg, B. S. J. Derrubone, an inhibitor of the Hsp90 protein folding machinery. J. Nat. Prod. 2007, 70, 2014-2018.
11. Hadden, M. K.; Blagg, B. S. J. Cytotoxic small molecule dimers and their inhibitory activity against human breast cancer cells. Bioorg. Med. Chem. Lett. 2007, 17, 5063-5067.
10. Galam, L.; Hadden, M. K.; Ma, Z.; Ye, Q-Z.; Yun, B-G.; Blagg, B. S. J.; Matts, R. L. High-throughput assay for the identification of Hsp90 inhibitors based on Hsp90-dependent refolding of firefly luciferase. Bioorg. Med. Chem. 2007, 15, 1939-1946.
9. Ansar, S.; Burlison, J. A.; Hadden, M. K.; Yu, X. M.; Desino, K. E.; Bean, J.; Neckers, L.; Audus, K. A.; Michaelis, M. L.; Blagg, B. S. J. A non-toxic Hsp90 inhibitor protects neurons from Aβ-induced toxicity. Bioorg. Med. Chem. Lett. 2007, 17, 1984-1990.
8. Hadden, M. K.; Lubbers, D. J.; Blagg, B. S. J. Geldanamycin, radicicol, and chimeric inhibitors of the Hsp90 N-terminal ATP binding site. Curr. Top. Med. Chem. 2006, 6, 1173-1182.
7. Avila, C.; Hadden, M. K.; Ma, Z.; Kornilayev, B. A.; Ye, Q. Z.; Blagg, B. S. J. High-throughput screening for Hsp90 ATPase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 3005-3008.
6. Orwig, K. S.; Lassetter, M. R.; Hadden, M. K.; Dix, T.A. Comparison of N-terminal modifications of neurotensin (8-13) analogues correlates peptide stability but not binding affinity with in vivo efficacy. J. Med. Chem. 2009, 52, 1803-1813.
5. Hadden, M. K.; Orwig, K. S.; Kokko, K. P.; Mazella, J.; Dix, T. A. Design, synthesis, and evaluation of the antipsychotic potential of orally bioavailable neurotensin(8-13) analogues containing non-natural arginine and lysine residues. Neuropharmacology 2005, 49, 1149-1159.
4. Hadden, M. K.; Kokko, K. P.; Dix, T. A. Asymmetric synthesis of w-bromo-2(S)-methyl acids as precursors for novel arginine, lysine, and mercapto residues. Syn. Comm. 2005, 35, 1675-1680.
3. Hadden, M. K.; Walle, T.; Dix, T. A. Cellular uptake of a radiolabelled analogue of neurotensin in the caco-2 cell model. J. Pharm. and Pharmacol. 2005, 57, 327-333.
2. Kokko, K. P.; Hadden, M. K.; Price, K. L.; Orwig, K. S.; See, R. E.; Dix, T. A. Behavioral effects of stable, receptor-selective neurotensin[8-13] analogues that cross the blood-brain barrier. Neuropharmacology 2003, 48, 417-425.
1. Kokko, K. P.; Hadden, M. K.; Orwig, K. S., Mazella, J.; Dix, T. A. In vitro analysis of stable, receptor-selective neurotensin[8-13] analogues. J. Med. Chem. 2003, 46, 4141-4148.