Mammalian embryogenesis is a complex, highly regulated process that requires both temporal and spatial control of multiple cellular signals to ensure proper cellular growth, tissue differentiation, and organ development. Many of these processes are controlled through several evolutionarily conserved developmental signaling cascades, which include the Hedgehog (Hh), Notch, and Wnt pathways. Various ligands and receptors in these pathways act as morphogens to regulate distinct developmental processes, the functional effects of which depend on the morphogen present and the overall cellular context. Based on their essential roles during embryogenesis, the activity of these pathways is tightly regulated by multiple mechanisms including feedback inhibition, small molecule activation and inhibition, and crosstalk between the pathways. One class of endogenous small molecules that have been implicated as a regulatory mechanism for all three of these pathways is the vitamin D class of seco-steroids; however, the exact mechanisms through which this class of compounds exerts these effects is poorly understood.
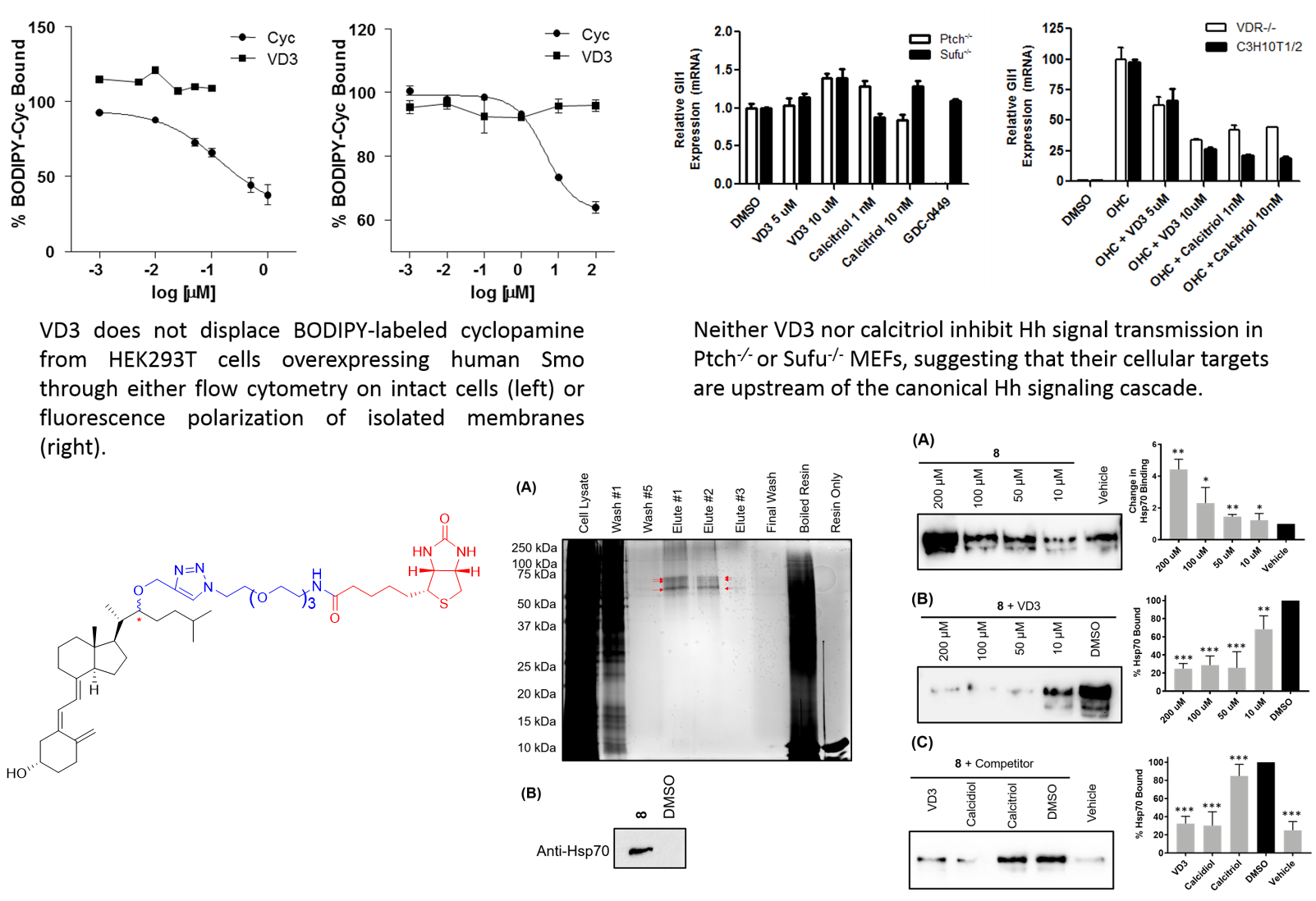
The classic physiological activity associated with the vitamin D scaffold is maintaining calcium and phosphorous homeostasis in the bone. This activity is commonly attributed to direct binding of 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3, calcitriol], the hormonally active form of vitamin D, to the vitamin D receptor (VDR). More recently, calcitriol and VDR have been shown to control the expression of genes associated with cellular proliferation and differentiation in a wide variety of cells, suggesting more extensive biological activity for the vitamin D system. The effects of vitamin D-based seco-steroids with respect to developmental signaling has primarily focused on the ability of both calcitriol and its metabolic precursor vitamin D3 (VD3) to inhibit Hh signaling. Initial studies in this area suggested that both calcitriol and VD3 inhibited Hh signal transmission at the level of Smoothened (Smo), a 7-transmembrane GPCR-like receptor that is a key Hh pathway component. By contrast, follow-up studies in our laboratory are inconsistent with calcitriol and VD3 functioning to inhibit Hh signaling via either Smo or VDR.
It is important to note that all of the previous studies exploring seco-steroid regulation of Hh signaling have focused on indirect methods of identifying the cellular mechanisms that govern vitamin D regulation of Hh signaling. The Hadden lab is currently engaged in several series of experiments specifically designed to explore direct binding interactions between calcitriol, VD3, and potential cellular binding factors that could mediate the ability of these endogenous compounds to regulate Hh signaling. Recently, these studies identified Hsp70 as a novel binding target of VD3 and calcitriol and demonstrated that Hsp70 plays a role in regulating the Hh signaling pathway.