The Hedgehog Pathway
Inhibition of the hedgehog (Hh) pathway represents an exciting new intervention strategy toward the development of cancer chemotherapeutics due to its integral role in the development of a variety of human cancers.
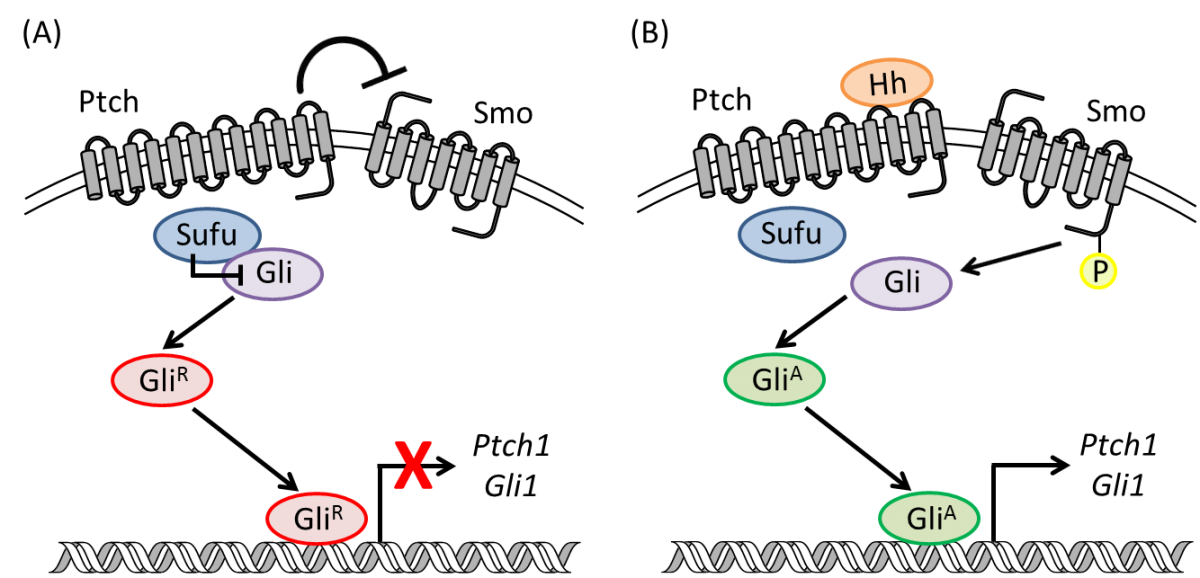
Mammalian embryogenesis is a complex, highly regulated process that requires both temporal and spatial control of multiple cellular signals to ensure proper cellular growth, tissue differentiation, and organ development. Many of these processes are controlled through several evolutionarily conserved developmental signaling cascades, which include the Hedgehog (Hh), Notch, and Wnt pathways. Various ligands and receptors in these pathways act as morphogens to regulate distinct developmental processes, the functional effects of which depend on the morphogen present and the overall cellular context. Based on their essential roles during embryogenesis, the activity of these pathways is tightly regulated by multiple mechanisms including feedback inhibition, small molecule activation and inhibition, and crosstalk between the pathways.
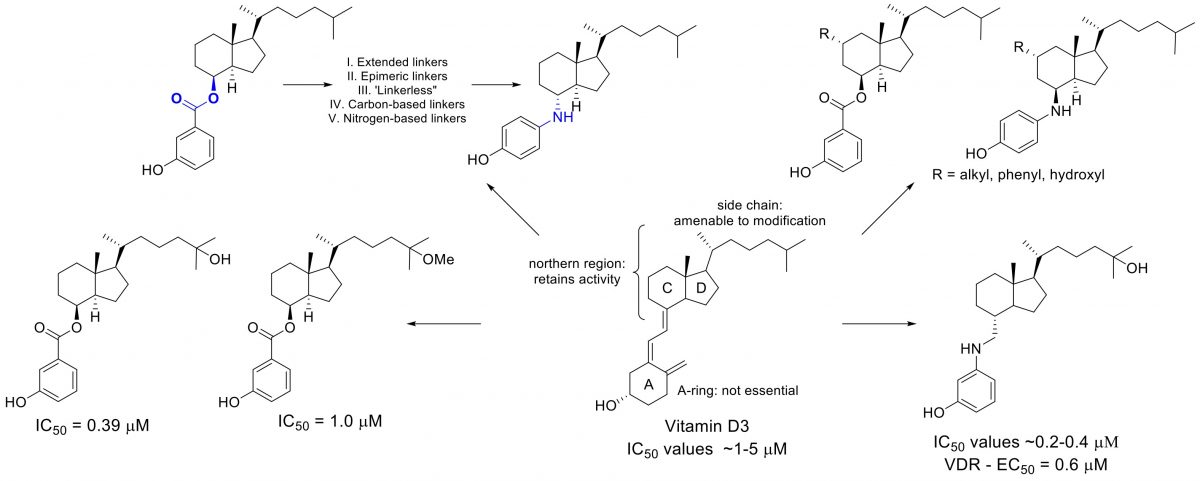
Vitamin D3 Analogues as Hedgehog Pathway Inhibitors
Recent years have seen a marked increase in research aimed at the potential preventative and therapeutic anti-cancer effects of vitamin D. Much of this interest has focused on 1α,25-dihydroxyvitamin D3 (calcitriol), generally acknowledged as the hormonally active form of vitamin D. These effects are primarily mediated through the vitamin D receptor (VDR), a nuclear receptor that acts as a transcription factor to regulate apoptosis, proliferation, and differentiation. However, the clinical usefulness of calcitriol and analogues has been limited by its tendency to promote hypercalcemia, an effect related to its VDR-mediated role in calcium regulation.
Recent studies have demonstrated that vitamin D3, a metabolic precursor to calcitriol, inhibits the hedgehog signaling pathway by direct binding to smoothened, a key regulatory protein in the hedgehog signal cascade. Inhibition of this pathway represents an exciting new intervention strategy toward the development of cancer chemotherapeutics due to its integral role in the development of skin and brain cancers. The development of vitamin D3 analogues rationally designed to exert their anti-cancer effects through inhibition of hedgehog signaling would be less susceptible to the detrimental side-effects associated with calcitriol and represent an improved class of vitamin D-based anti-cancer chemotherapeutics.
We found that it was difficult to find a balance between potent and selective Hh inhibition, and that there is a role for multiple proteins (SMO, VDR, TGFβR, and Hsp70) in mediating anti-Hh activity of VD3.
Relevant Publications
Banerjee, U.; Ghosh, M.; Hadden, M. K. Evaluation of vitamin D3 A-ring analogues as hedgehog pathway inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1330-1334.
DeBerardinis, A.; Banerjee, U.; Miller, M.; Lemieux, S.; Hadden, M. K. Probing the structural requirements for vitamin D3 inhibition of hedgehog signaling. Bioorg. Med. Chem. Lett. 2012, 22, 4859-4863.
DeBerardinis, A. M.; Banerjee, U.; Hadden, M. K. Identification of vitamin D3-based hedgehog pathway inhibitors that incorporate an aromatic A-ring isostere. ACS Med. Chem. Lett. 2013, 4, 590-595.
DeBerardinis, A. M.; Madden, D. J.; Banerjee, U.; Sail, V.; Raccuia, D. S.; De Carlo, D.; Lemieux, S. M.; Meares, A.; Hadden, M. K. Structure-activity relationships for vitamin D3-based aromatic A-ring analogues as hedgehog pathway inhibitors. J. Med. Chem. 2014, 57, 3724-3736.
Banerjee, U.; DeBerardinis, A. M.; Hadden, M. K. Design, synthesis, and evaluation of hybrid vitamin D3 side chain analogues as hedgehog pathway inhibitors. Bioorg. Med. Chem. 2015, 23, 548-555
DeBerardinis, A. M.; Raccuia, D. S.; Maschinot, C. A.; Thompson, E.; Hadden, M. K. Vitamin D3 analogues that contain modified A- and seco-B-rings as hedgehog pathway inhibitors. Euro. J. Med. Chem. 2015, 93, 156-171.
Maschinot, C. A. and Hadden, M. K. Synthesis and evaluation of vitamin D3 analogus with C-11 modifications as inhibitors of hedgehog signaling. Bioorg. Med. Chem. Lett. 2017, 27, 4011-4014.
Wen, J. and Hadden, M. K. Structure–Activity Relationship Studies of Vitamin D3 Analogues Containing an Ether or Thioether Linker as Hedgehog Pathway Inhibitors. ChemMedChem 2018, 13, 748-753.
Maschinot C. A.; Chau, L. Q.; Wechsler-Reya, R. J.; Hadden, M. K. Synthesis and evaluation of third generation vitamin D3 analogues as inhibitors of hedgehog signaling. J. Med. Chem. 2019, 162, 495-506.
Wen, J.; Hadden, M. K. Affinity-based protein profiling identifies vitamin D3 as a heat shock protein 70 antagonist that regulates hedgehog transduction in murine basal cell carcinoma. J. Med. Chem. 2022, 228, 114005.
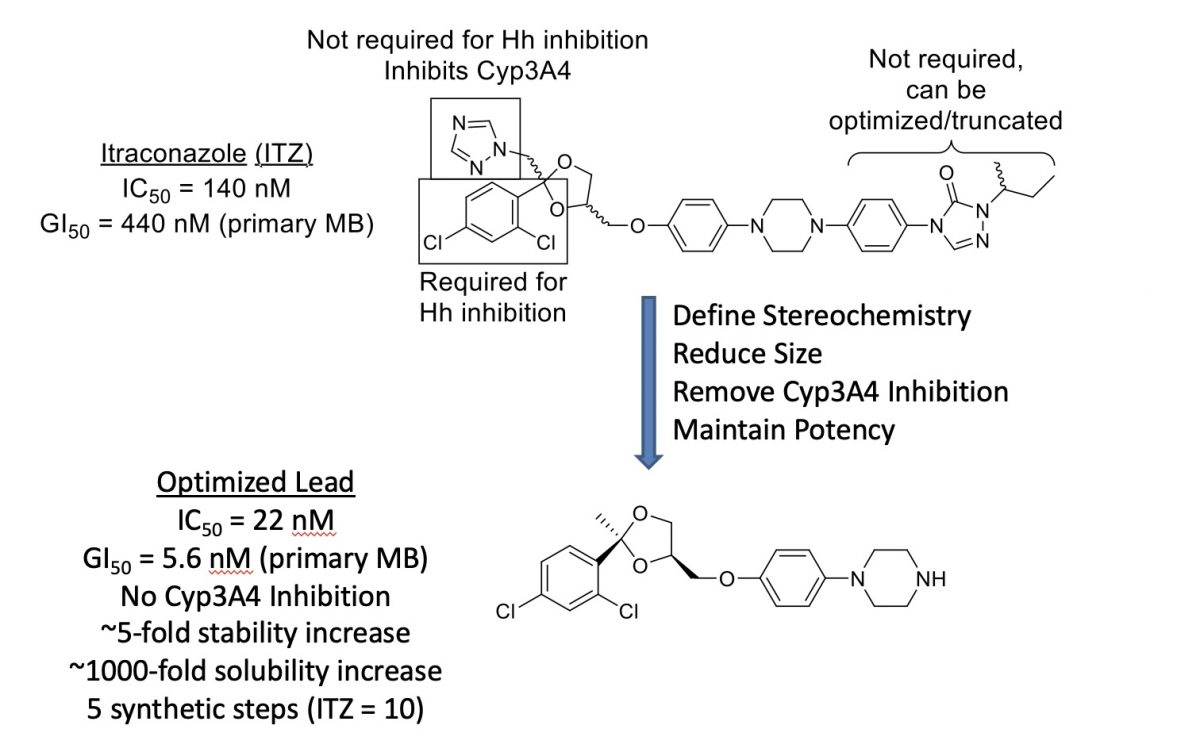
Repurposing Itraconazole as an Anti-Cancer Agent
Itraconazole (ITZ) is a member of the triazole class of antifungal agents that exerts its antimycotic effects through potent inhibition of lanosterol 14α-demethylase (14LDM, also known as CYP51), a cytochrome P450 enzyme that catalyzes the oxidative conversion of lanosterol to ergosterol. Recent studies have shown that ITZ also functions as a potent inhibitor of both Hh signaling and angiogenesis in vitro and in vivo (IC50 values ~100-700 nM, depending on assay and cell type). Most importantly, ITZ maintains inhibitory activity against multiple forms of Smo (including D473H) that confer resistance to GDC-0449 and prolongs survival of mice with GDC-0449 resistant forms of medulloblastoma. Preliminary structure-activity relationship (SAR) studies for ITZ-mediated Hh inhibition and angiogenesis performed in the Hadden lab have identified multiple regions of the ITZ scaffold that are amenable to modification. Our data suggests that further modifications to these regions will provide ITZ analogues that maintain potent Hh inhibition, while also demonstrating improved pharmacokinetic (PK) parameters and reduced off-target side effects characteristic of ITZ treatment.
We identified and optimized the lead compound below:
Relevant Publications
Pace, J. R.; DeBerardinis, A. M.; Sail, V.; Tacheva-Grigorova, S. K.; Chan, K. A.; Tran, R.; Raccuia, D. S.; Wechsler-Reya, R.; Hadden, M. K. Repurposing the clinically efficacious anti-fungal agent itraconazole as an anti-cancer chemotherapeutic. J. Med. Chem. 2016, 59, 3635-3649.
Teske, K. A.; Dash, R. C.; Morel, S. R.; Chau, L. Q.; Wechsler-Reya, R. J.; Hadden, M. K. Development of posaconazole-based analogues as hedgehog signaling pathway inhibitors. Euro. J. Med. Chem. 2019, 163, 320-332.
Pace, J. R.; Teske, K. A.; Chau, L. Q.; Dash, R. C.; Zaino, A. M.; Wechsler-Reya, R. J.; Hadden, M. K. Structure-activity relationships for itraconazole-based triazolone analogues as hedgehog pathway inhibitors. J. Med. Chem. 2019, 62, 3873-3885.
Wen, J. Chennamadhavuni, D.; Morel, S. R.; Hadden, M. K. Truncated itraconazole analogues exhibiting potent anti-hedgehog activity and improved drug-like properties. ACS Med. Chem. Lett. 2019, 10, 1290-1295.
Pace, J. R. ; Jog, R.; Burgess, D. J.; Hadden, M. K. Formulation and evaluation of itraconazole liposomes for hedgehog pathway inhibition. J. Liposome Res. 2020, 30, 305-311.
Wen, J.; Teske, K. A.; Hadden, M. K. Inhibition of hedgehog signaling by stereochemically defined des-triazole itraconazole analogues. Bioorg. Med. Chem. Lett. 2020, 30, 126794.