The Role of m6A Modification Proteins
Epitranscriptomics, the field of post-translational RNA modifications, is a burgeoning domain of research that has recently received significant attention for its role in multiple diseases, including cancer. N6-methyladenosine (m6A) is the most prominent post-translational RNA modification and plays a critical role in RNA transcription, processing, translation, and metabolism. The m6A modification is controlled by three protein classes known as writers (methyltransferases), erasers (demethylases), and readers (m6A-binding proteins). Each class of m6A regulatory proteins has been implicated in cancer initiation and progression. As such, many of these proteins have been identified as potential targets for anti-cancer chemotherapeutics. Interestingly, each of these regulator proteins have been identified as both oncogenic and tumor suppressive, depending on the cancer subtype or tissue of origin.
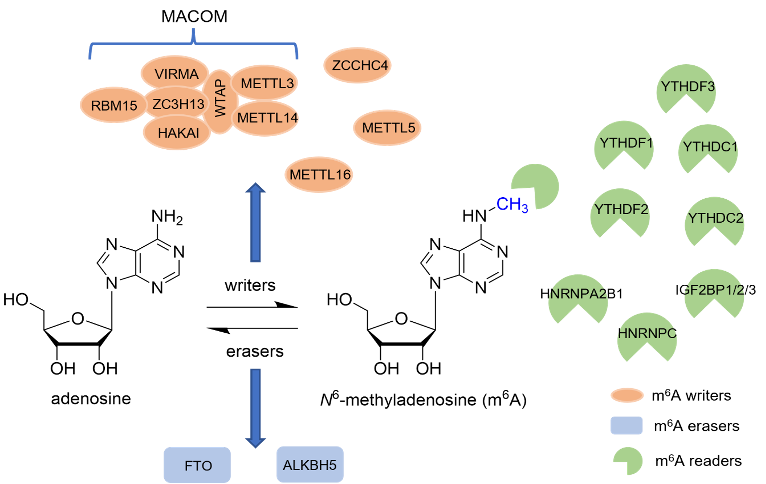
The m6A residue mediates a wide range of cellular effects and disruption of its normal functions has been implicated as a potential driver for the development and/or progression of several diseases, such as cancer, neuronal disorders, and metabolic diseases. As such, the writers, erasers, and readers that govern m6A installation and recognize the modification in order to regulate these various cellular processes have been implicated as potential drug targets. Some of the most-studied m6A regulators include METTL3/METTL14 (m6A methyltransferases), FTO and ALKBH5 (m6A demethylases), and YTH Domain Proteins (m6A readers).
Several recent review articles have provided thorough recaps and in-depth analysis of the cellular mechanisms through which each of these proteins contribute to tumor development. Our work aims to further explore these m6A regulators and the primary mechanisms they utilize to govern tumor onset and progression.
Relevant Publications
Harrahill, N. J. and Hadden, M. K. Small molecules that regulate the N6-methyladenosine RNA modification as potential anti-cancer agents. J. Med. Chem. 2024, 274, 116526.