Translesion Synthesis Inhibitors as Anti-Cancer Agents
Translesion synthesis (TLS) is a DNA damage tolerance mechanism that replaces the replicative DNA polymerase with a specialized, low-fidelity TLS DNA polymerase that can copy past DNA lesions during active replication. Recent studies have demonstrated a primary role for TLS in replicating past DNA lesions induced by first-line genotoxic agents, resulting in decreased efficacy and acquired chemoresistance. With this in mind, targeting TLS as a combination strategy with first-line genotoxic agents has emerged as a promising approach to develop a new class of anti-cancer adjuvant agents.
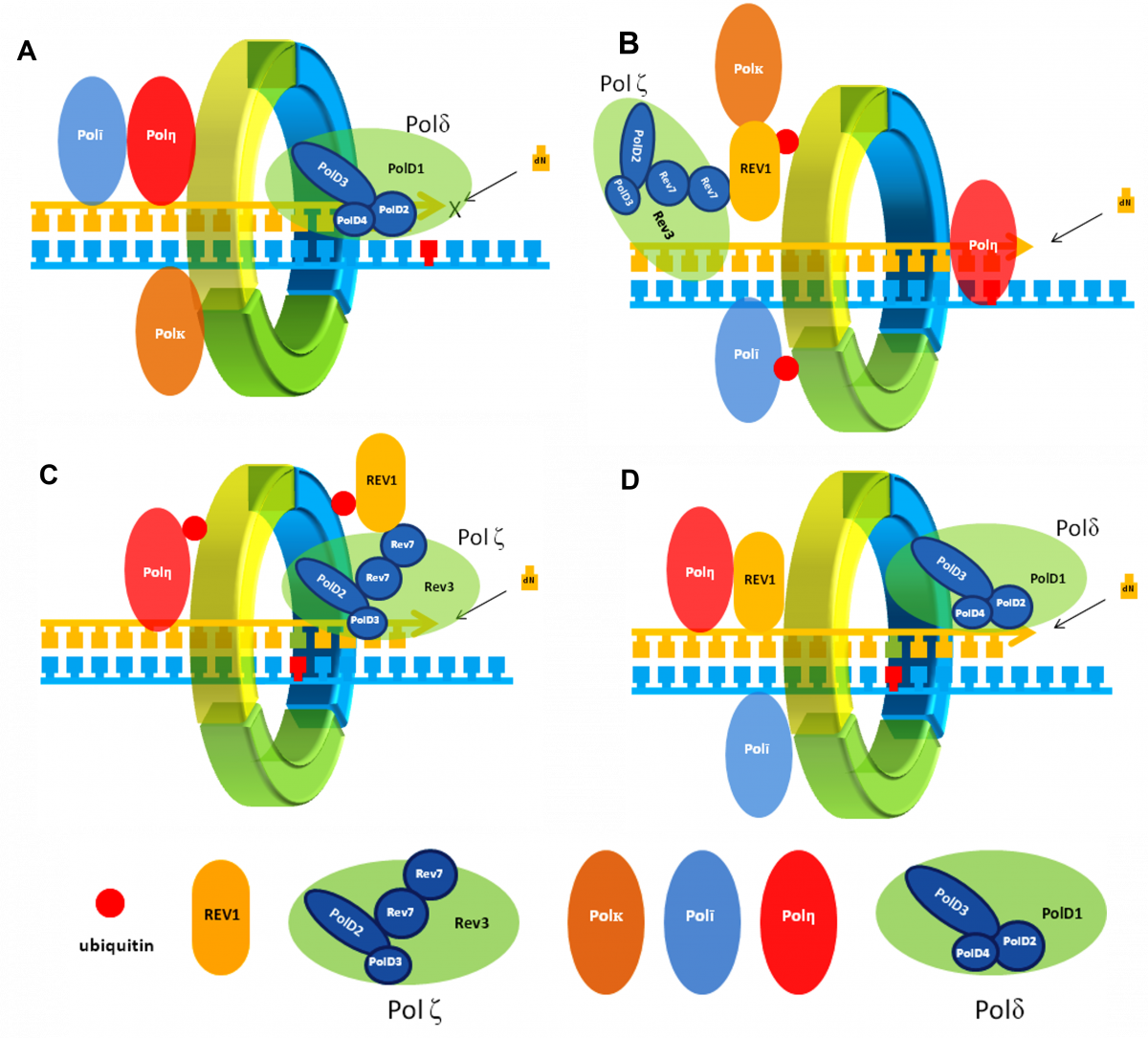
The replicative bypass of bulky DNA adducts caused by these first-line agents is mediated by a set of specialized low-fidelity TLS DNA polymerases that can copy over DNA lesions while temporarily leaving them unrepaired. Multi-protein complexes that act in this process are comprised of the Y-family DNA polymerases Rev1, polη, polι and/or polκ and the B-family polymerase polζ assembled on a DNA-sliding clamp PCNA. Because TLS is inherently mutagenic, cells have evolved a complicated network of protein-protein interactions (PPIs) that regulate TLS function to ensure that the TLS heteroprotein complex is only recruited to sites of DNA damage. More specifically, these PPIs fine-tune assembly of the TLS complex, they localize the proper TLS DNA polymerase to the lesion site, and they regulate polymerase switching events between replicative and TLS polymerases. These PPIs also allow the TLS complex to adopt different configurations depending on the type of DNA lesion, where the lesion occurs, and what specific step in TLS is currently underway. The Hadden lab is utilizing multiple approaches to identify small molecules that disrupt several key TLS PPIs and develop them as anti-cancer agents.
REV1-CT/RIR Interaction
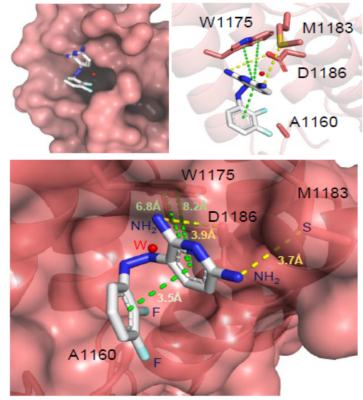
REV1 plays important structural and regulatory roles in TLS, with its primary function to serve as a scaffold for assembly of the multiprotein TLS complex and regulate recruitment of other TLS polymerases. REV1 binds with the REV1 interacting regions (RIRs) from the Y-family polymerases POLη, POLι, and POLκ and the POLD3 subunit of the B-family polymerase POLζ, allowing for the recruitment of inserter and extender polymerases to the lesion site. Inhibitors of this interaction is a promising avenue for the development of TLS inhibitor molecules.
REV7/3 Interaction
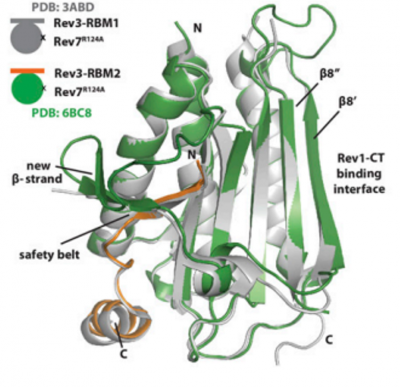
REV7, a subunit of POLζ, is an essential connector protein in TLS polymerase recruitment and switching. REV7 serves as a bridge by interacting with both REV1 and REV3. Upon REV7/3 binding, a conformational change is induced that allows REV1-CT to interact with REV7, leading to the formation of a complete REV1/POLζ complex. Subsequently, the POLζ complex can carry out its primary function to extend the primer DNA past the nucleotide inserted by one of the Y-family polymerases at a DNA damage site. Thus, targets of the REV7/3 interaction are hopeful avenues to explore to attenuate TLS activity.
Relevant Publications
Korzhnev, D. M. and Hadden, M. K. Targeting the translesion synthesis pathway for the development of anti-cancer chemotherapeutics. J. Med. Chem. 2016, 59, 9321-9336.
Dash, R. C.; Ozen, Z.; Rizzo, A. A.; Lim, S.; Korzhnev, D. M.; Hadden, M. K. Structural approach to identify a lead scaffold that targets the translesion synthesis polymerase Rev1. J. Chem. Inf. Model. 2018, 58, 2266-2277.
Patel, S. M.; Dash, R. C.; Hadden, M. K. Translesion synthesis inhibitors as a new class of cancer chemotherapeutics. Invited Review. Expert Opin. Inv. Drugs. 2021, 30, 13-24.
Dash, R. C.; Hadden, M. K. Protein-protein interactions in translesion synthesis. Molecules 2021, 26, 5544.