REV1-CT/RIR Interactions in Translesion Synthesis
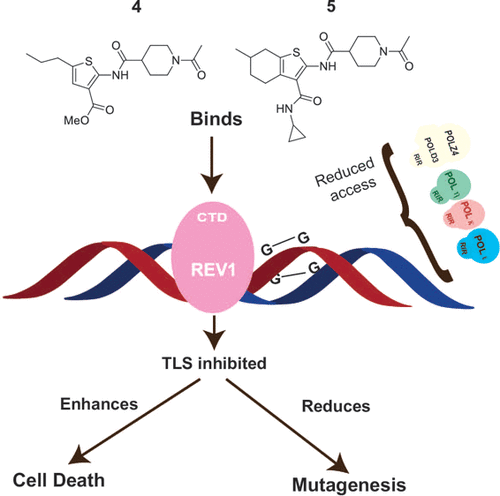
Translesion synthesis (TLS) is an important mechanism through which proliferating cells tolerate DNA damage during replication. The mutagenic Rev1/Polζ-dependent branch of TLS helps cancer cells survive first-line genotoxic chemotherapy and introduces mutations that can contribute to the acquired resistance so often observed with standard anticancer regimens. As such, inhibition of Rev1/Polζ-dependent TLS has recently emerged as a strategy to enhance the efficacy of first-line chemotherapy and reduce the acquisition of chemoresistance by decreasing tumor mutation rate. The TLS DNA polymerase Rev1 serves as an integral scaffolding protein that mediates the assembly of the active multiprotein TLS complexes. Protein–protein interactions (PPIs) between the C-terminal domain of Rev1 (Rev1-CT) and the Rev1-interacting region (RIR) of other TLS DNA polymerases play an essential role in regulating TLS activity.
We have designed a fluorescence polarization-based assay to be utilized as a pilot screen for small molecule inhibitors of this PPI, and identified multiple small molecule scaffolds that disrupt this interaction. Upon further survival and mutagenesis assays in mouse embryonic fibroblasts and human fibrosarcoma HT1080 cells treated with cisplatin and ultraviolet light, we found that our identified small molecule compounds inhibit mutagenic Rev1/Polζ-dependent TLS in cells, validating the Rev1-CT/RIR PPI for future anticancer drug discovery and identifying the first small molecule inhibitors of TLS that target Rev1-CT. In addition, through a series of biochemical, computational, and cellular studies, we have identified preliminary structure–activity relationships and determined initial pharmacokinetic parameters for our hits.
Relevant Publications
Sail, V.; Rizzo, A. A.; Chatterjee, N.; Dash, R. C.; Ozen, Z.; Walker, G. C.; Korzhnev, D. M.; Hadden, M. K. Identification of small molecule translesion synthesis inhibitors that target the Rev1-CT/RIR protein-protein interaction. ACS Chem. Biol. 2017, 12, 1903-1912.
Ozen, V.; Dash, R. C.; McCarthy, K. R.; Chow, S. A.; Rizzo, A. A.; Korzhnev, D. M.; Hadden, M. K. Small molecule scaffolds that disrupt the Rev1-CT/RIR protein-protein interaction. Bioorg. Med. Chem. 2018, 26, 4301-4309.
Dash, R. C.; Ozen, Z.; Rizzo, A. A.; Lim, S.; Korzhnev, D. M.; Hadden, M. K. Structural approach to identify a lead scaffold that targets the translesion synthesis polymerase Rev1. J. Chem. Inf. Model. 2018, 58, 2266-2277.
Dash, R. C.; Ozen, Z.; McCarthy, K. R.; Chatterjee, N.; Harris, C. A.; Rizzo, A. A.; Walker, G. C.; Lorzhnev, D. M.; Hadden, M. K. Virtual pharmacophore screening identifies small-molecule inhibitors of the Rev1-CT/RIR protein-protein interaction. ChemMedChem 2019, 14, 1610-1617.
Nayak, S.; Calvo, J. A.; Cong, K.; Peng, M.; Berthiaume, E.; Jackson, J.; Zaino, A. M.; Vindigni, A.; Hadden, M. K.; Cantor, S. B. Inhibition of the translesion synthesis polymerase Rev1 exploits replication gaps as a cancer vulnerability. Sci. Adv. 2020, 6, eaaz7808.
McPherson, K. S.; Zaino, A. M.; Dash, R. C.; Rizzo, A. A.; Li, Y.; Hao, B.; Bezsonova, I.; Hadden, M. K.; Korzhnev, D. M. Structure-based drug design of phenazopyridine derivatives as inhibitors of Rev1 interactions in translesion synthesis. ChemMedChem 2021, 16, 1126-1132.
Ikeh, K. E.; Lamkin, E. N.; Crompton, A.; Deutsch, J.; Fisher, K. J.; Gray, M.; Argyle, D. J.; Lim, W. Y.; Korzhnev, D. M.; Hadden, M. K.; Hong, J.; Zhou, P.; Chatterjee, N. REV1 inhibition enhances radioresistance and autophagy. Cancers 2021, 13, 5290.
Zaino, A. M.; Dash, R. C.; James, S. J.; MacGilvary, N.; Crompton, A.; McPherson, K. S.; Stanzione, M.; Korzhnev, D. M.; Dyson, N. J.; Chatterjee, N.; Cantor, S. B.; Hadden, M. K.; Lead compound profiling for small molecule inhibitors of the REV1-CT/RIR Translesion synthesis Protein-Protein interaction. Bioorg Med Chem. 2024, 106, 117755.